Abstract
Background
Pre implant heart failure severity may affect post implant health-related quality of life (HRQOL). The purpose of our study was to examine differences in HRQOL from before mechanical circulatory support (MCS) through 1 year after surgery, by INTERMACS patient profiles.
Methods
Data from adult patients with advanced heart failure who received primary continuous flow pumps between 6/23/06 – 3/31/10 and were enrolled in INTERMACS (n=1,559) were analyzed. HRQOL data were collected using the EQ-5D-3L survey pre implant and at 3, 6 and 12 months after implant. Statistical analyses included chi square and t-tests, using all available data for each time period. Paired ttests and sensitivity analyses were also conducted.
Results
Quality of life was poor before MCS implant among patients with INTERMACS profiles 1–7 and significantly improved after MCS for all profiles. Stratified by INTERMACS profile, problems within each of the five dimensions of HRQOL (i.e., mobility, self-care, usual activities, pain, and anxiety / depression) generally decreased from before to after implant. By six months after implant, patients with all INTERMACS profiles reported similar frequencies of problems for all HRQOL dimensions. Paired ttests and sensitivity analyses supported the vast majority of our findings.
Conclusions
HRQOL is poor among advanced heart failure patients with INTERMACS profiles 1–7 before MCS implantation and improves to similar levels for patients who remained on MCS 1 year after surgery. Patients have problems in HRQOL dimensions before and after MCS; the frequency of reporting problems decreases for all dimensions within most profiles across time.
The severity of heart failure has traditionally been characterized using the New York Heart Association (NYHA) functional classification system and gauges the severity of symptoms for patients who have American Heart Association Stage C or D heart failure.1 Patients with advanced heart failure with NYHA Class III or IV symptoms have limitations in daily activities despite optimal medical management. However, not all patients with advanced heart failure have a similar prognosis. Investigators of the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS, a prospective registry that collects data on all patients who receive a durable, FDA approved mechanical circulatory support [MCS] device in the United States) developed a classification system of patient profiles to further characterize the severity of heart failure in this high risk population of patients undergoing treatment with MCS.2 Seven patient profiles (1=critical cardiogenic shock to 7=advanced NYHA Class III symptoms) were defined based on clinical descriptors at the time of MCS implant.2, 3
INTERMACS profiles have been used to characterize risk for poor outcomes after MCS implantation. Stepanenko et al.,4 in their study on outcomes of elective versus emergent permanent (i.e., destination therapy) MCS in elderly patients with advanced heart failure, reported that patients not requiring inotrope support (INTERMACS profiles 4–7) had lower operative mortality than patients with cardiogenic shock on high dose inotropes (INTERMACS profiles 1–3), cautiously concluding that permanent MCS may benefit highly selected elderly patients. In another study, patients with INTERMACS profiles 4–7 had better actuarial survival and a shorter hospital length of stay than patients with INTERMACS profile 1.5 Kirklin et al.6 reported that INTERMACS profile 1 is a risk factor for death early after MCS implantation as destination therapy.
Regarding patient-reported health related quality of life (HRQOL) outcomes, researchers have demonstrated improved HRQOL through as long as 2 years after MCS implantation for both bridge to transplant and destination therapy.7–10 However, improvement in HRQOL from before to after MCS implantation across the spectrum of heart failure severity is unknown. Given that pre-implant heart failure severity affects post implant clinical outcomes, it may affect post implant HRQOL outcomes as well. Determining MCS HRQOL benefits and risks, stratified by heart failure severity, may provide important information with which to inform patients who are considering MCS implantation, and thereby help patients make more informed health care decisions, given that severely ill patients make decisions on treatment options (e.g., including surgical therapies or continued medical therapy / palliative care / hospice), based on outcomes, including HRQOL, as well as survival.11–14 Such information may also guide development of more targeted strategies to enhance HRQOL after implant. Understanding HRQOL benefits of MCS is especially important for patients who receive MCS as destination therapy. In the future, indications for destination therapy MCS may expand to include patients with moderately advanced heart failure, thus providing further impetus for understanding variation in post MCS HRQOL outcomes by pre-implant advanced heart failure severity.15
The purpose of our study was to examine differences in HRQOL outcomes from before MCS implantation through 1 year after surgery among patients receiving continuous flow pumps, stratified by INTERMACS patient profiles. We defined HRQOL as “the functional effect of an illness and its consequent therapy upon a patient, as perceived by the patient.”16
METHODS
Sample
The source of data for this study was INTERMACS. Study inclusion criteria were: (1) adult patients (age ≥19 at time of implant), (2) who received a primary implant between June 23, 2006 and March 31, 2010, and (3) whose left ventricular assist device (LVAD) was a continuous flow pump. Exclusion criteria were patients (n=9) from four hospitals that did not collect post-implant HRQOL data. The follow-up date for this study was March 31, 2011, allowing the opportunity for each patient to have 1 year of post-implant follow-up. These criteria resulted in a sample of 1,559 patients from 101 hospitals. Patients were categorized according to INTERMACS profiles (Table 1).
Table 1. Description of INTERMACS PROFILES.
Severity of advanced heart failure in patients being considered for mechanical circulatory support has been characterized as per the INTERMACS* profiles.
| PROFILE | Description |
|---|---|
| 1 | Critical cardiogenic shock |
| - life threatening hypotension and rapidly escalating inotropic pressor support | |
| 2 | Progressive decline |
| - dependent on inotropic support, with signs of deterioration | |
| 3 | Stable but inotrope dependent |
| - clinically stable on mild-moderate doses of intravenous inotropes | |
| 4 | Resting symptoms |
| - at home on oral therapy, but frequently has symptoms of congestion | |
| 5 | Exertion intolerant |
| - comfortable at rest but unable to engage in any activity | |
| 6 | Exertion limited |
| - comfortable at rest without evidence of fluid overload, able to do mild activity | |
| 7 | Advanced NYHA Class 3 |
| - clinically stable with a reasonable level of comfortable activity | |
| Stevenson et al., JHLT’10 | |
INTERMACS = Interagency Registry for Mechanically Assisted Circulatory Support
Instruments
HRQOL was measured using the EQ-5D-3L survey, a generic, self-report HRQOL instrument including five questions measuring the following dimensions of HRQOL: mobility, self-care, usual activities, pain / discomfort, and anxiety / depression.17, 18 For each dimension, patients choose among three levels of Likert scaled responses: no problems; some or moderate problems; and extreme problems. The EQ-5D also has a newer 5 level response version for the 5 dimensions (EQ-5D-5L) that is more precise, which has been available since April, 2011. Since we have 5 years of INTERMACS data using the 3 level response version and determined that it is not appropriate to map from a 3 level response format to a 5 level response format, we chose to continue to use the EQ-5D-3L. The EQ-5D-3L also includes an overall health status rating, using a vertical visual analog scale (VAS), with 0 = worst imaginable health state and 100 = best imaginable health state. Psychometric support for this instrument has been reported.19 Data from medical records, including demographic and behavioral characteristics and pre-implant clinical characteristics, were also collected.
Procedures
All sites participating in INTERMACS received Institutional Review Board approval. Patients provided consent and were subsequently enrolled in the registry. For this report, EQ-5D-3L data and medical records data were collected by research coordinators pre-implant and at 3, 6, and 12 months post-implant until device removal, transplant, or death during this 12 month follow-up period. Patients completed the EQ-5D as close to the implant date as possible, up to 30 days prior. Reasons for missing data were recorded when the EQ-5D-3L was not completed. Data were entered electronically into the INTERMACS database and analyzed by the data coordinating center, located at the University of Alabama, Birmingham, AL.
Statistical Analyses
Patients with INTERMACS profiles 5, 6, and 7 were combined into one group due to small sample sizes in each group and sufficient similarity in severity of illness (i.e., being exertion intolerant, exertion limited, or advanced NYHA class III). Data were analyzed using SAS, version 9.1 (Carey, NC). The EQ-5D-3L VAS score was reported as a mean + standard deviation, and dimension scores were reported as frequencies. Analyses for dimensions were dichotomized (no problems vs. moderate or extreme problems), and frequencies of moderate and extreme problems were displayed graphically in figures. Statistical analyses included chi square to compare proportions and t-tests to compare means, using all available data for each time period. Paired t-tests were used to test the robustness of our analyses for a subset of patients with complete pre and 12 month post implant HRQOL data. Level of significance was p < 0.05 for longitudinal comparisons between pre implant and 12 month post implant time periods, as well as for cross-sectional comparisons for all profiles within a given time period.
Data from the five dimensions were clustered into two groups: (group 1) physical function / activities of daily living (mobility, self-care, and usual activities) and (group 2) pain / emotions (pain / discomfort and anxiety / depression). This grouping was used because a response level of “Extreme problems” was assigned to patients who were too sick to respond (as reported by the patient or research coordinator) for the physical function / activities of daily living group. This response level was assigned post hoc to reduce the potential for overestimation of HRQOL in patients who were most severely ill. No assignment of responses was made for too sick patients for the pain / emotions group, as being too sick does not necessarily indicate extreme problems regarding pain or negative emotions. A VAS rating of 0 was assigned to patients who also were too sick to respond. We performed sensitivity analyses to assess the influence of assignment of scores for patients too sick to respond.
RESULTS
Description of cohort
There were 1,559 advanced HF patients enrolled in INTERMACS pre implant between 2006 and 2010 who received a primary continuous flow pump. All patients included in this report had pre implant EQ-5D-3L data (or a value assigned if too sick to respond) and post implant EQ-5D data at one or more time periods. Pre implant, group 1 (i.e., the physical function / activities of daily living group) included 1190/1559 (76.3%) patients. The majority of group 1 patients were white, middle-aged men who were married and well educated (Table 2). Before implant, the vast majority of these patients were in INTERMACS profiles 1–3 (84%), and only 5% of patients were in INTERMACS profiles 5–7. Group 2 patients (i.e., the pain / emotions group) (n=822 pre implant) had similar demographic and clinical characteristics as group 1 (data not displayed); however, included fewer INTERMACS profile 1 patients.
Table 2.
Characteristics of pre implant MCS recipients with and without EQ-5D-3L data
| Pre-Implant Characteristics | Pre implant MCS recipients who completed the EQ-5D n=1190* |
Pre implant MCS recipients who did not complete the EQ-5Dn=369 |
p-value |
|---|---|---|---|
| Demographic and behavioral characteristics | |||
| Age at implant (mean years) | 53.48 | 54.11 | 0.39 |
| Male (%) | 77.2 | 80.5 | 0.19 |
| Race (% white) | 69.4 | 65.9 | 0.20 |
| Married at time of implant (%) | 62.3 | 62.3 | 1.00 |
| > high school education (%) | 52.6 | 53.5 | 0.79 |
| Currently smoking (%) | 13.2 | 12.0 | 0.59 |
| Current alcohol abuse (%) | 15.2 | 18.3 | 0.16 |
| Current drug abuse (%) | 2.5 | 3.0 | 0.66 |
| Clinical characteristics | |||
| Primary cardiac diagnosis (%) | |||
| Ischemic cardiomyopathy | 36.1 | 35.0 | 0.68 |
| Dilated cardiomyopathy | 49.7 | 56.8 | 0.04 |
| Other | 14.2 | 9.2 | 0.01 |
| Co-morbidities (%) | |||
| Diabetes | 37.9 | 40.1 | 0.46 |
| CVA | 8.1 | 5.3 | 0.08 |
| Right heart failure (RVEF severe) | 24.3 | 26.3 | 0.60 |
| Pre COPD | 12.6 | 14.8 | 0.30 |
| NYHA class IV (%) | 79.6 | 71.1 | 0.0014 |
| Intra aortic balloon pump (%) | 36.0 | 31.2 | 0.09 |
| Ventilator (%) | 9.7 | 4.1 | 0.0007 |
| ECMO | 2.4 | 1.4 | 0.21 |
| Dialysis | 2.1 | 2.7 | 0.49 |
| INTERMACS profile at implant (%) | |||
| 1 | 18.5 | 11.4 | 0.0014 |
| 2 | 45.9 | 40.4 | 0.06 |
| 3 | 19.8 | 25.5 | 0.02 |
| 4 | 10.7 | 13.0 | 0.21 |
| 5 | 2.4 | 4.6 | 0.03 |
| 6 | 1.7 | 3.0 | 0.12 |
| 7 | 1.0 | 2.2 | 0.0002 |
| Device strategy (%) | |||
| Bridge to transplant –listed | 46.8 | 48.0 | 0.70 |
| Bridge to transplant –likely to be listed | 30.5 | 33.1 | 0.35 |
| Bridge to transplant –moderately likely to be listed | 9.9 | 9.2 | 0.69 |
| Bridge to transplant –unlikely to be listed | 3.4 | 2.2 | 0.25 |
| Destination therapy | 7.9 | 6.5 | 0.38 |
| Inotrope therapy (%) | 83.6 | 74.8 | 0.0002 |
| Implantable cardioverter defibrillator (%) | 78.7 | 83.0 | 0.07 |
| Temporary circulatory support (%) | 12.9 | 11.6 | 0.56 |
MCS = mechanical circulatory support, CAD = coronary artery disease, CVA = cerebrovascular accident, COPD = chronic obstructive pulmonary disease, ECMO = extracorporeal membrane oxygenation
Those patients who were ‘too sick’ have been included in the ‘completed’ column and assigned VAS=0 and physical dimensions as ‘extreme problems’ (i.e., group 1)
Differences in pre implant clinical characteristics were detected for group 1 patients with EQ-5D-3L data (n=1190) versus patients without EQ=5D-3L data who were not included in our study (n=369) (Table 2). Pre implant, more group 1 patients were NYHA class IV and on inotrope therapy and a ventilator than patients without EQ-5D-3L data. There were no differences between groups regarding demographic characteristics, behavioral variables, and co-morbidities.
EQ-5D-3L survey completion rates overall and by INTERMACS patient profile
Rates of survey completion were also examined for group 1 and group 2 patients by INTERMACS patient profiles. Data are displayed for group 1 patients in table 3; data are not displayed for group 2 patients. Differences in rates of survey completion were detected for both group 1 and group 2 patients across time for all INTERMACS profiles, except for INTERMACS profiles 5–7. There were also differences in rates of survey completion by INTERMACS profiles within the pre implant and 6 month post implant time periods for group 1 and 2 patients. Common reasons for lack of survey completion were administrative (e.g., patient not consented, no contact with the patient during the window of time that a survey was due) and patient refusal to participate.
Table 3.
Adult Primary Continuous Flow LVAD Patients who completed the EQ-5D-3L by Patient Profile at: Pre-Implant, 3 months, 6 months and 12 months post implant
| Post-implant |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Implant |
3 months |
6 months |
12 months |
||||||||||
| Patient Profile | n | QOL* | % | n | QOL* | % | n | QOL* | % | n | QOL* | % | p-value |
| Level 1 | 262 | 220 | 84.0% | 235 | 113 | 48.1% | 211 | 73 | 34.6% | 141 | 49 | 34.8% | < 0.0001 |
| Level 2 | 695 | 546 | 78.6% | 649 | 323 | 49.8% | 590 | 251 | 42.5% | 425 | 172 | 40.5% | < 0.0001 |
| Level 3 | 330 | 236 | 71.5% | 322 | 149 | 46.3% | 301 | 133 | 44.2% | 228 | 96 | 42.1% | <0.0001 |
| Level 4 | 175 | 127 | 72.6% | 163 | 78 | 47.9% | 155 | 67 | 43.2% | 110 | 54 | 49.1% | <0.0001 |
| Levels 5–7 | 97 | 61 | 62.9% | 91 | 44 | 48.4% | 83 | 45 | 54.2% | 66 | 30 | 45.5% | 0.1 |
| Total | 1559 | 1190 | 76.3% | 1460 | 707 | 48.4% | 1340 | 569 | 42.5% | 970 | 401 | 41.3% | |
| P value | < 0.0001 | 0.89 | 0.03 | 0.21 | |||||||||
Note: The INTERMACS patient profile level is collected prior to implant. This table illustrates the breakdown of patient profile level for those patients who completed the EQ-5D questionnaire at the specified time points. The p-values in the last column reflect differences in rates of completion for each INTERMACS profile across time. The p-values in the last row reflect differences in rates of completion for each INTERMACS profile at a specified time period.
QOL = Patients who answered the EQ-5D-3L (Note: Those patients who did not complete the EQ-5D because they were ‘too sick’ were assigned the VAS=0 and the physical dimensions as extreme problems and were included in this table as EQ-5D form completions).
Differences in HRQOL among INTERMACS profiles before and after MCS implantation
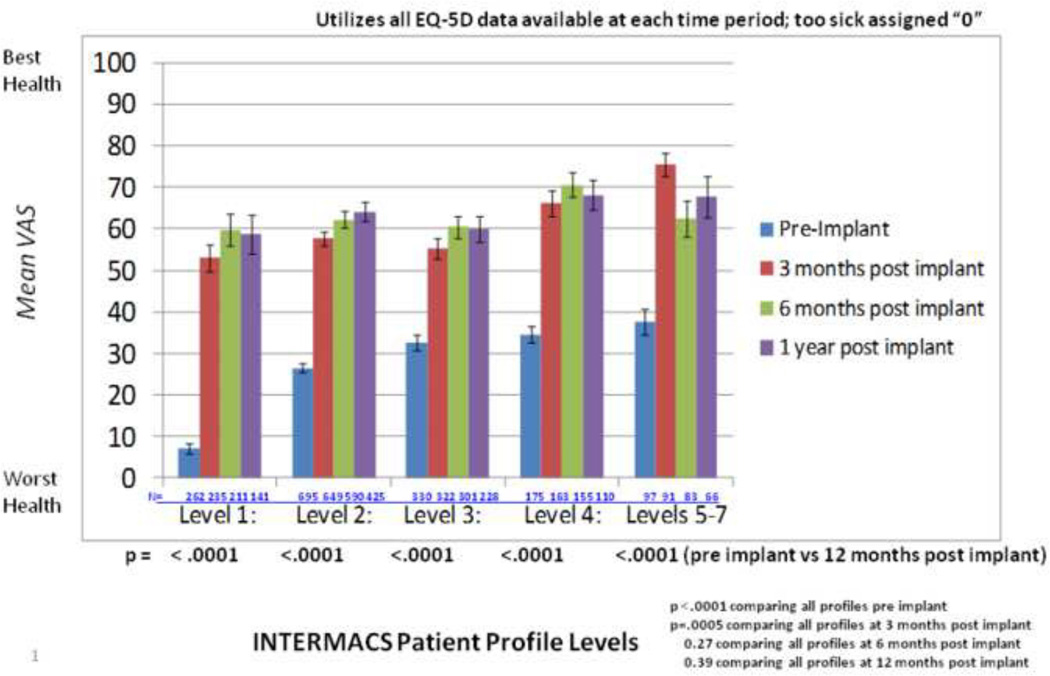
Differences in HRQOL were examined by INTERMACS profile from baseline through 12 months after implantation. Prior to MCS, mean VAS scores revealed poor health status among patients with all INTERMACS profiles, although scores differed significantly by profile (Figure 1). Patients with all INTERMACS profiles experienced significantly improved health status from before to 12 months post implant. Health status differed significantly among profiles at 3 months after implant but was similar for all profiles at 6 and 12 months after implant.
Figure 1.
EQ-5D: Visual Analog Scale
Physical Function /Activities of Daily Living Dimensions (group 1)
Stratified by INTERMACS profile, patient problems within dimensions of HRQOL generally decreased from before to after implantation. Before implantation, the majority of patients with all INTERMACS profiles had problems with mobility (≥ 70%), and usual activities (≥ 80%), while significantly fewer patients with all profiles had problems 12 months after implant (Figure 2A–B). Although the frequency of problems with self care before implant varied significantly among profiles, significantly fewer patients with all profiles had problems by 12 months after implant, except profiles 5–7 (figure 2C).
Figure 2.
Physical Function / Activities of Daily Living Dimensions
Differences in frequency of reporting problems by INTERMACS profile were also examined cross-sectionally for group 1 patients within each time period. Before implantation, significant differences in problems with mobility, usual activities, and self-care were detected among the profiles (figure 2A–C). At 3, 6, and 12 months after implantation, frequency of problems were generally similar for all profiles.
The presence of extreme versus some / moderate problems for the HRQOL dimensions is displayed graphically in figure 2A–C. Although no formal analyses were conducted, empirically, more patients with INTERMACS profile 1 had extreme problems versus some problems regarding mobility, usual activities, and self-care than patients with other profiles before implantation.
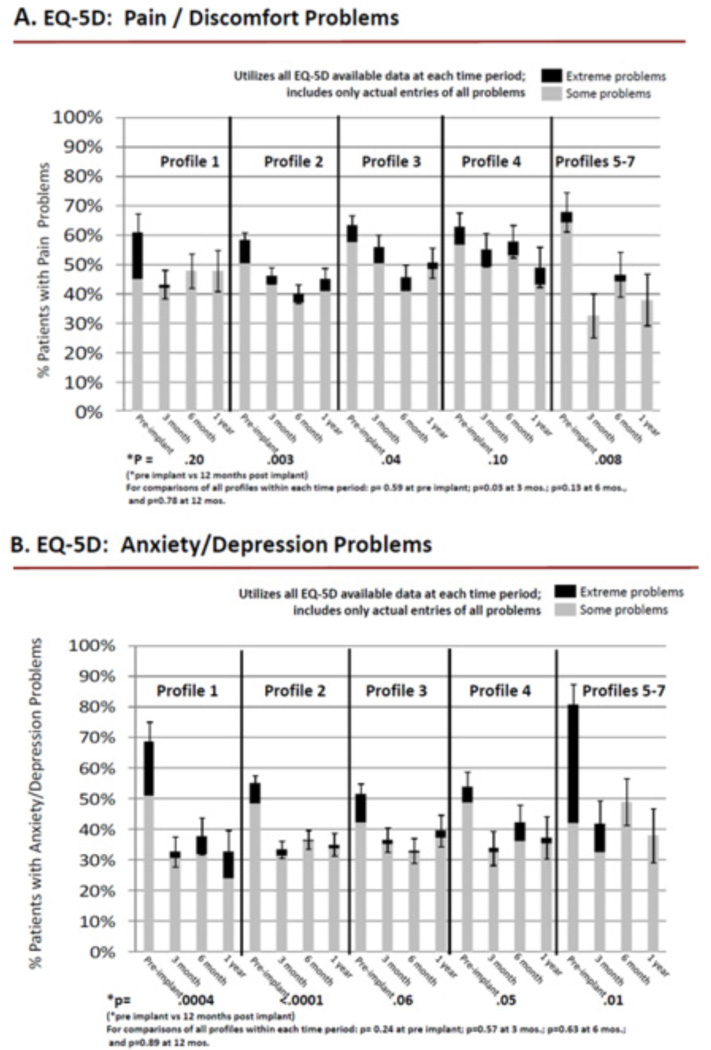
Pain/Emotion Dimensions (group 2)
More than 50% of patients with all INTERMACS profiles reported having problems with pain before implantation, and significantly fewer patients reported pain at 12 months after implantation, except for patients with INTERMACS profiles 1 and 4 (Figure 3A). The majority of patients with all INTERMACS profiles reported anxiety / depression before implant, which decreased significantly at 12 months post implantation, except for profile 3, wherein there was a strong trend (Figure 3B).
Figure 3.
Pain / Emotion Dimensions
Differences in frequency of reporting problems by INTERMACS profile were also examined for group 2 patients cross-sectionally within each time period. There were almost no differences in frequency of reporting of pain and anxiety / depression before implantation and at 3, 6, and 12 months after implantation (Figure 3A–B).
Regarding empirically observed differences in levels of problems by INTERMACS profile for group 2 patients, most patients reported moderate problems, rather than extreme problems for pain and anxiety / depression before and after implant (figure 3A–B). Notably, almost one half of patients with INTERMACS profiles 5–7 reported extreme problems with anxiety / depression before implantation.
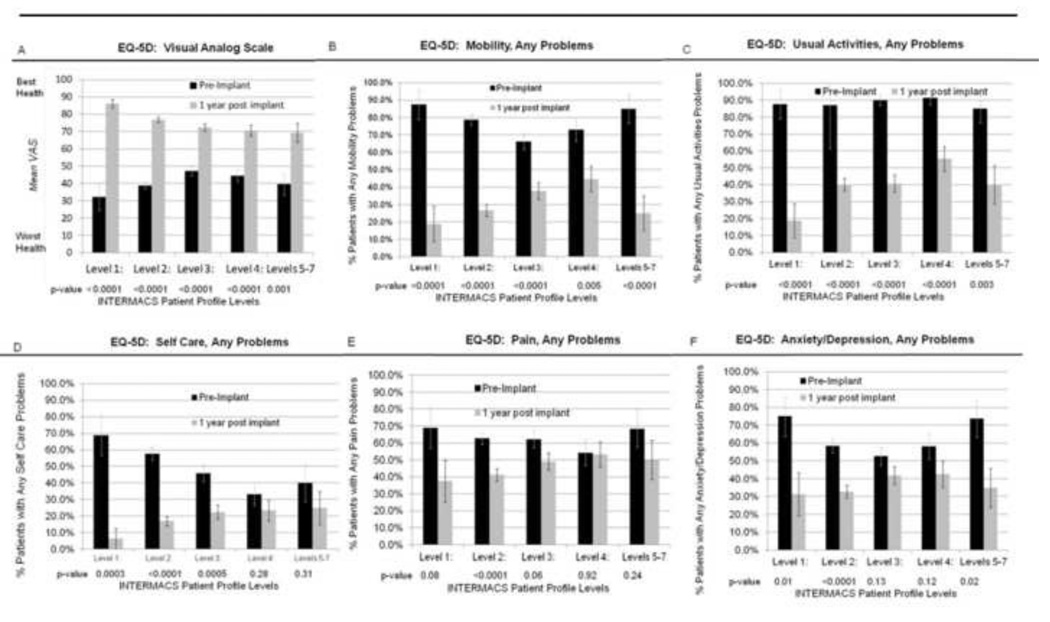
Comparisons for MCS patients with complete data
Comparisons using paired data before and at 12 months post implant (n=348) supported the vast majority of our findings. We found a similar pattern of significant increase in overall health status and decrease in problems for all five dimensions for the vast majority of profiles across time (figure 4A–F). These paired comparisons did not reveal significant decreases in problems from pre to 12 months post implantation for INTERMACS profiles 4 and 5–7 regarding self-care (figure 4D), for INTERMACS profiles 3, 4, and 5–7 regarding pain (figure 4E), and for INTERMACS profiles 3 and 4 regarding anxiety / depression (figure 4F).
Figure 4.
Comparisons for MCS patients with complete data
Includes only patients with a complete EQ-5D prior to implant AND at 1 year post implant, n=348
Sensitivity analyses
Sensitivity analyses, without prospective assignment of missing data for patients too sick to respond, resulted in similar findings for the VAS and all three dimensions, mobility, usual activities, and self-care, thus supporting our findings.
DISCUSSION
Our findings provide important new information regarding advanced heart failure patients with the highest levels of disease severity and worst prognoses among patients with cardiovascular disease. Advanced heart failure patients with all INTERMACS profiles had poor overall HRQOL at baseline, although HRQOL scores differed among profiles. One year after surgery, HRQOL levels had improved, and patients with all profiles had similar levels of HRQOL. Frequency of problems for all five dimensions of HRQOL decreased significantly from before to 1 year after implantation for patients with all INTERMACS profiles, with few exceptions. Similar to overall HRQOL, frequencies of problems did not differ by INTERMACS profile for all five HRQOL dimensions at 12 months after implantation.
The vast majority of our findings were supported by both paired t-tests and use of all available data. Specifically, using both methods of analyses, we found significant improvement in health status and decrease in problems with mobility and usual activities for all profiles; decrease in problems with self-care for profiles 1, 2, and 3; decrease in problems with pain for profile 2, and decrease in problems with anxiety/depression for profiles 1, 2, and 5–7. Also, a lack of significance for decreases in problems were found using paired t-tests as well as all available data for self-care (profiles 5–7), pain (profiles 1 and 4), and anxiety/depression (profiles 3 and 4). Significant differences were detected when using all available data, while no significant differences were detected using paired t-tests only for decreases in problems for self-care (profile 4) and pain (profiles 3 and 5–7). Thus, paired t-tests supported the robustness of our analyses using all available data.
To our knowledge, this is the first report that examines HRQOL by heart failure severity from before to after MCS implantation. Prior reports have described overall improvement in HRQOL from before to early and later after implantation, for first and second generation devices.8, 9, 20–22 HRQOL outcomes by preoperative heart failure severity have also been examined in patients after heart transplantation.23 We previously reported that patients, regardless of pre transplant heart failure severity, were quite satisfied with their overall HRQOL at 6 months after transplantation and reported similar and low levels of disability regarding mobility, self-care, and / or emotional status. Our findings in an MCS cohort are similar to these heart transplant patients.
However, MCS patients, no matter what profile, have worse overall HRQOL both before and after implant as compared to a normative U.S. adult (≥ 18 years) general population (U.S adult mean VAS score = 82).24 Furthermore, the vast majority of the U.S. adult population frequently report no problems in the five EQ-5D dimensions (range=74–96%).25 Our findings, when examined within the context of the U.S. adult population, reveal the profound impact of advanced heart failure on HRQOL, and yet also demonstrate improvement in HRQOL for all profiles after MCS.
Studies of HRQOL, using the EQ-5D, in other chronic illness populations provide additional insight into our findings. Calvert et al.26 reported low EQ-5D VAS scores in primarily NYHA class III heart failure patients (range of mean VAS scores, stratified by age = 45–55). These mean VAS scores were not as low as mean scores in our cohort, even for patients at higher INTERMACS profiles. Using the EQ-5D, Almenar-Pertejo et al., reported a pre heart transplant mean VAS score = 37, and a mean VAS score range = 66 – 71 through 1 year after surgery, similar to mean scores for patients in our study.27 Patients from this study also reported significant improvement in all HRQOL dimensions, except for pain, from before to after heart transplantation.
Our findings demonstrate that regardless of disease severity, HRQOL improves from before to 1 year after MCS implantation. Understanding specific problems reported by patients, no matter what disease severity, provides guidance for clinicians to monitor potential areas of concern and develop interventions to enhance HRQOL both before and after MCS. For example, > 30% of all patients reported pain/discomfort and anxiety/depression from baseline through 1 year after MCS. While fewer patients with some INTERMACS profiles reported problems in these dimension after MCS, patients with other profiles reported similar levels of problems both before and after MCS. These findings require further exploration, with a more sensitive “problem-specific” instrument. Regarding pain, it would be helpful to assess location of pain, extent of pain, degree of relief from pain with treatment, and the interference of pain with activities of daily living, sleep, etc. Regarding location of pain, given that post MCS EQ-5D surveys are completed no sooner than 3 months after implant, we would not anticipate reports of pain related to a sternotomy or driveline tunneling, but perhaps related to an infection at the driveline exit site. Similarly, anxiety and depression may be mild, moderate, or severe and further assessment with an instrument specific to each diagnosis would be useful to guide treatment options. Also, it would be important to understand an individual patient’s clinical trajectory (e.g., pre, early or later post MCS) and implant strategy regarding psychological problems. For example, patients may be anxious about learning self-care early after MCS, while other patients may become depressed if they experience a major complication later after MCS. Furthermore, bridge to transplant patients may experience anxiety and / or depression regarding uncertainty about the timing of transplant, while destination therapy patients may experience anxiety and / or depression about their length of life on MCS.
Our findings also provide insight into the HRQOL of patients with less severe heart failure. Given that patients with INTERMACS profiles 5–7 have poor overall health status, and problems with mobility, usual activities, and anxiety and / or depression pre implantation which improve significantly 12 months after implantation, our findings support investigation of HRQOL outcomes in patients with moderately advanced heart failure across time.15
Our study has limitations. HRQOL data were collected using a brief generic health profile. A heart failure-specific HRQOL instrument may have provided additional information. However, it is also important to note that in a recent review article, Dyer et al.28 reported adequate psychometric support (including validity, reliability and responsiveness) for use of the EQ-5D as an outcome measure within the cardiovascular area, including heart failure. There was significant attrition of patients by 12 months after surgery due to transplantation, death, recovery, device exchange, and transfer of care, which may have also influenced our findings. Also, the sample size was small for patients with INTERMACS profiles 5–7 at 12 months after implant. Additionally, there were missing data. We prospectively assigned responses for the VAS and three of five dimensions for patients who were too sick to respond to the EQ-5D, which may have reduced overestimation of HRQOL across time. Sensitivity analyses, without prospective assignment of missing data for patients too sick to respond, supported our findings. We plan to evaluate these analytic decisions in the future.
CONCLUSION
HRQOL is poor among patients with advanced heart failure with INTERMACS profiles 1–7 before MCS implantation and improves to similar levels for all patients by 1 year after surgery. Monitoring HRQOL-related problems and developing interventions may enhance HRQOL for all patients, regardless of disease severity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
K Grady- PI, AHA grant-in-aid; PI, R34 NHLBI grant
David Naftel, PhD – consultant HeartWare and Thoratec Corporations
Mary Amanda Dew, PhD: no disclosures
Gerdi Weidner, PhD – supported by grants from the Alexander-von-Humboldt Foundation
Susan Myers, BA – no disclosures
Francis D. Pagani, MD, PhD– National Co-PI ENDURANCE trial
James K Kirklin, MD – no disclosures
Lynne Stevenson, MD – no disclosures
Timothy Baldwin, PhD – no disclosures
James Young, MD – no disclosures
References
- 1.Braunwald E, Zipes D, Libby P, Bonow R, editors. Braunwald’s Heart Disease: A textbook of cardiovascular medicine. Philadelphia, PA: Elsevier, Inc.; 2005. [Google Scholar]
- 2.Stevenson LW, Pagani FD, Young JB, et al. INTERMACS profiles of advanced heart failure: The current picture. J Heart Lung Transplant. 2009;28:535–541. doi: 10.1016/j.healun.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Kirklin JK, Naftel DC, Kormos R, et al. The Fourth INTERMACS Annual Report: 4,000 implants and counting. J Heart Lung Transplant. 2012;31:117–136. doi: 10.1016/j.healun.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Stepanenko A, Potapov E, Jurmann B, et al. Outcomes of elective versus emergent permanent mechanical circulatory support in the elderly: A single-center experience. J Heart Lung Transplant. 2010;29:61–65. doi: 10.1016/j.healun.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Boyle AJ, Ascheim DD, Russo MJ, et al. Clinical outcomes for continuous-flow left ventricular assist device patients stratified by pre-operative INTERMACS classification. J Heart Lung Transplant. 2011;30:402–407. doi: 10.1016/j.healun.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Kirklin JK, Naftel DC, Kormos RL, et al. Third INTERMACS Annual Report: The evolution of destination therapy in the United States. J Heart Lung Transplant. 2011;30:115–123. doi: 10.1016/j.healun.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Grady KL, Meyer PM, Dressler D, Mattea A, Chillcott S, Loo A, et al. Longitudinal change in quality of life and impact on survival after left ventricular assist device implantation. Ann Thorac Surg. 2004;77(4):1321–1327. doi: 10.1016/j.athoracsur.2003.09.089. [DOI] [PubMed] [Google Scholar]
- 8.Pagani FD, Miller LW, Russell SD, Aaronson KD, John R, Boyle AJ, et al. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol. 2009;54(4):312–321. doi: 10.1016/j.jacc.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 9.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, et al. Advanced Heart Failure Treated with Continuous-Flow Left Ventricular Assist Device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 10.Rogers J, Aaronson KD, Boyle AJ, et al. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol. 2010;55:1826–1834. doi: 10.1016/j.jacc.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 11.Field M, Cassel C, editors. Approaching Death: Improving care at the end of life. Washington DC: Institute of Medicine, National Academy Press.; 1997. [PubMed] [Google Scholar]
- 12.MacIver J, Rao V, Delgado D, et al. Choices: A study of preferences for end-of-life treatments in patients with advanced heart failure. J Heart Lung Transplant. 2008;27:1002–1007. doi: 10.1016/j.healun.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Moskowitz A, Weinberg A, Oz M, Williams D. Quality of life with an implanted left ventricular assist device. Ann Thorac Surg. 1997;64:1764–1769. doi: 10.1016/s0003-4975(97)01000-x. [DOI] [PubMed] [Google Scholar]
- 14.Allen L, Stevenson L, Grady K, et al. Decision making in advanced heart failure: A statement from the American Heart Association. Circ. 2012;125(15):1928–1952. doi: 10.1161/CIR.0b013e31824f2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baldwin T, Mann D. NHLBI’s Program for VAD Therapy for Moderately Advanced Heart Failure: The REVIVE-IT Pilot Trial. J Cardiac Fail. 2010;16:855–858. doi: 10.1016/j.cardfail.2010.06.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spilker B. Quality of life and pharmacoeconomics in clinical trials. 2nd ed. New York: Lippincott Williams & Williams; 1996. [Google Scholar]
- 17.EuroQol group. EuroQol a new facility for the measurement of health related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 18.Rabin R, de Charro F. EQ-5D: A measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 19.van Agt H, Essink-Bot ML, Krabbe P, Bonsel G. Test-retest reliability of health state valuations collected with the EuroQoL questionnaire. Soc Sci Med. 1994;39:1537–1544. doi: 10.1016/0277-9536(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 20.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med. 2001;345(20):1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 21.Park SJ, Tector A, Piccioni W, Raines E, Gelijns A, Moskowitz A, Rose E, Holman W, Furukawa S, Frazier OH, Dembitsky W. Left ventricular assist devices as destination therapy: A new look at survival. J Thorac Cardiovasc Surg. 2005;129:9–17. doi: 10.1016/j.jtcvs.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 22.Grady KL, Meyer P, Mattea A, White-Williams C, Ormaza S, Kaan A, et al. Improvement in quality of life outcomes 2 weeks after left ventricular assist device implantation. J Heart Lung Transplant. 2001;20(6):657–669. doi: 10.1016/s1053-2498(01)00253-4. [DOI] [PubMed] [Google Scholar]
- 23.Grady KL, Jalowiec A, White-Williams C. Quality of life 6 months after heart transplantation compared with indicators of illness severity before transplantation. Am J Crit Care. 1998;7:106–116. [PubMed] [Google Scholar]
- 24.Johnson J, Coons SJ. Comparison of the EQ5-D and SF-12 in an adult U.S. sample. Quality Life Res. 1998;7:155–166. doi: 10.1023/a:1008809610703. [DOI] [PubMed] [Google Scholar]
- 25.Luo N, Johnson J, Shaw J, et al. Self-reported health status of the general adult U.S. population as assessed by the EQ-5D and Health Utilities Index. Medical Care. 2005;43(11):1078–1086. doi: 10.1097/01.mlr.0000182493.57090.c1. [DOI] [PubMed] [Google Scholar]
- 26.Calvert MJ, Freemantle N, Cleland J. The impact of chronic heart failure on health-related quality of life data acquired in the baseline phase of the CARE-HF study. Euro J Heart Failure. 2005;7:243–251. doi: 10.1016/j.ejheart.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Almenar-Pertejo M, Almenar L, Martinez-Dolz L, et al. Study on health-related quality of life in patients with advanced heart failure before and after transplantation. Transplant Proc. 2006;38:2524–2526. doi: 10.1016/j.transproceed.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 28.Dyer M, Goldsmith K, Sharples L, Buxton M. A review of health utilities using the EQ-5D in studies of cardiovascular disease. Health and Quality of Life Outcomes. 2010;8:13. doi: 10.1186/1477-7525-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]