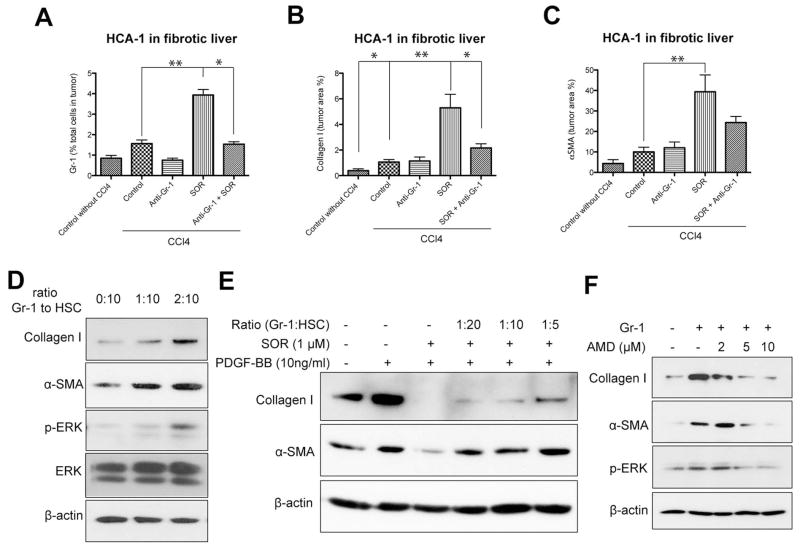
Figure 6. Gr-1+ myeloid cell infiltration increases fibrosis in HCC after sorafenib in an SDF1α/CXCR4 pathway-dependent manner.
A–C, Depletion of Gr-1+ myeloid cells using systemic therapy with anti-Gr-1 blocking antibodies prevents the shift towards a pro-fibrotic environment after sorafenib treatment. Sorafenib significantly increased the number of 7AAD–CD11b+Gr1+ monocytes (A) (N=3–7 mice per group), the collagen I content (B) and the number of α-SMA+ myofibroblasts (C) in orthotopic HCA-1 tumors growing in mice with liver fibrosis, evaluated by immunohistochemistry. Combining anti-Gr-1 antibodies with sorafenib treatment prevented these effects. Quantification was performed in 5–12 confocal microscopy images per mouse. D–F, Tumor-infiltrating Gr-1+ myeloid cells directly mediate HSC differentiation to myofibroblasts via SDF1α/CXCR4 axis. Co-culture with tumor-tissue isolated Gr1+ myeloid cells (obtained using magnetic beads) increased collagen I and α-SMA expression levels as well as ERK activation in HSCs (D). Co-culture with Gr1+ myeloid cells increased collagen I and α-SMA expression in HSCs despite sorafenib treatment, in a dose-dependent manner (E). Recombinant PDGF-B and sorafenib alone were used as positive and negative control, respectively, for HSC differentiation (E). Inhibition of CXCR4 with AMD3100 prevented the increase in collagen I, α-SMA, and p-ERK expression in HSCs co-cultured with Gr-1+ myeloid cells (F). Data are presented as mean ± SEM. * P<0.05.