Abstract
Patients with cholestatic disease exhibit pruritus and analgesia, but the mechanisms underlying these symptoms are unknown. We report that bile acids, which are elevated in the circulation and tissues during cholestasis, cause itch and analgesia by activating the GPCR TGR5. TGR5 was detected in peptidergic neurons of mouse dorsal root ganglia and spinal cord that transmit itch and pain, and in dermal macrophages that contain opioids. Bile acids and a TGR5-selective agonist induced hyperexcitability of dorsal root ganglia neurons and stimulated the release of the itch and analgesia transmitters gastrin-releasing peptide and leucine-enkephalin. Intradermal injection of bile acids and a TGR5-selective agonist stimulated scratching behavior by gastrin-releasing peptide- and opioid-dependent mechanisms in mice. Scratching was attenuated in Tgr5-KO mice but exacerbated in Tgr5-Tg mice (overexpressing mouse TGR5), which exhibited spontaneous pruritus. Intraplantar and intrathecal injection of bile acids caused analgesia to mechanical stimulation of the paw by an opioid-dependent mechanism. Both peripheral and central mechanisms of analgesia were absent from Tgr5-KO mice. Thus, bile acids activate TGR5 on sensory nerves, stimulating the release of neuropeptides in the spinal cord that transmit itch and analgesia. These mechanisms could contribute to pruritus and painless jaundice that occur during cholestatic liver diseases.
Keywords: G-protein coupled receptors, cholestasis, pruritus, pain, neuron
Comment
Pruritus is a debilitating symptom of cholestatic liver disease.1 In adults, pruritus is commonly reported by patients with primary biliary cirrhosis2, and is an important feature of primary sclerosing cholangitis, and intrahepatic cholestasis of pregnancy. In children, severe pruritus is common in Alagille syndrome3, Progressive Familial Intrahepatic Cholestasis (particularly PFIC1 and PFIC2), and biliary atresia, and may be the indication for transplantation in these children. Moreover, the consequences of persistent, unrelenting, and poorly controlled pruritus can substantially impact patients’ quality of life. Given the related but antagonistic relationship between itch and pain4, it is not surprising that altered pain perception is also seen in cholestatic liver disease. Despite the magnitude of this clinical problem, progress towards understanding the pathogenesis of cholestatic pruritus has been slow. In the absence of clear molecular targets, current therapies remain largely empirical and are restricted to physical interventions such as nasobiliary drainage or use of pharmacological agents including bile acid sequestrants, rifampicin, opioid antagonist, and seretraline.5–7 The overall effectiveness of such interventions is limited, underscoring the substantial unmet need for these patients.
With regard to mechanism, it is believed that cholestasis results in hepatic release of unnamed pruritogens that act to stimulate specific neural itch fibers in the skin. This signal is then transmitted to neurons in the spinal cord, and ultimately to the brain. For many years, pruritus secondary to cholestasis was attributed to increased concentrations of bile acids and its role as a potential irritant in the skin. That contention arose from a variety of clinical and experimental observations, particularly studies where use of intermittent biliary drainage or bile acid sequestrants lowered serum bile acid concentrations and relieved pruritus in cholestatic patients.8,9 Although these clinical observations supported the concept that the pruritogen is most likely liver-derived, evidence was insufficient to directly implicate bile acids as the offending agent. Indeed, many other findings dating from the origin of the bile acid hypothesis argued strongly against a direct role of bile acids. Specifically, the most relevant counterargument is that not all cholestatic patients with markedly elevated serum bile acid concentrations, itch.10,11
So what are other candidate pruritogens besides bile acids? There is support for a role of altered endogenous opiate activity in cholestatic pruritus, a concept initially driven by the astute clinical observation of anti-pruritic actions of opiate antagonists.1,12 However, the mechanism does not appear to involve increased release of endogenous opioids by the cholestatic liver, but rather heightened opioidergic neurotransmission in response to cholestasis. In recent years, the most significant breakthrough in this area comes from a team of innovative investigators at the Academic Medical Center in Amsterdam, who provided compelling evidence that lysophosphatidic acid (LPA) is a mediator of cholestatic pruritus.13,14 The levels of LPA, a bioactive lipid previously shown to induce itch, rise in cholestatic patients as a result of increased levels of autotaxin, a circulating enzyme with lysophospholipase D enzyme activity.13,14 Evidence supporting a role for autotaxin and LPA in pruritus of cholestasis included a strong correlation between pruritus severity and autotaxin levels in patients before and following therapies such as bile acid sequestrants, rifampicin, and a Molecular Adsorbents Recirculation System (MARS). Notably, autotaxin levels were elevated in patients with pruritus of cholestatic origin, but not in other pruritus-associated conditions such as uremia or atopic dermatitis.14 Like many breakthroughs, the identification of LPA as a pruritogen in cholestasis has led to many more questions, including what is the source of autotaxin, the mechanisms responsible for its increased production, and the relationship of autotaxin/LPA to other candidate pruritogens. Joining our attempts to reconcile the evidence for bile acids, endogenous opioids, and now LPA in cholestatic pruritus is new and highly relevant evidence that bile acids can signal through a specific receptor, TGR5, to induce pruritus as well as analgesia.15
In addition to promoting hepatic bile secretion and intestinal absorption of fats and fat-soluble vitamins, it is clear that bile acids function as signaling molecules with effects that extend beyond control of hepatobiliary and intestinal function. Among the best studied of the bile acid-activated receptors is the G protein coupled receptor TGR5.16 Although not expressed by hepatocytes, TGR5 is expressed by many cell types including, surprising to some, neurons of the enteric and central nervous system, where it has been implicated in mediating the effects of bile acids on intestinal motility.17,18 In the present study, Alemi et al show that TGR5 is also expressed by the small diameter neurons of the dorsal root ganglia that are involved in transmission of both itch and pain signals from the skin. Addition of TGR5 agonists deoxycholic acid, the conjugated bile acid taurolithocholic acid, or oleanolic acid, a natural product found in the leaves of the European olive tree, stimulated action potentials in mouse dorsal root ganglion neurons as well as the release of neuropeptide mediators of itch (GRP; gastrin-releasing peptide) and analgesia (the endogenous opioid, leucine-enkephalin) from rat spinal cord. With regard to itch, intradermal injection of deoxycholic acid strongly stimulated scratching in wild type mice, and this response was stimulated in transgenic mice overexpressing TGR5. More remarkable was the finding that mice lacking TGR5 have substantially attenuated pruritic responses, suggesting a direct link between TGR5-activating bile acids and itch. In addition, with regard to pain, intraplantar or intrathecal injection of deoxycholic acid produced mechanical analgesia, which was dependent on the presence of TGR5 and blocked by opioid antagonists. As endogenous opioid-secreting dermal macrophages were shown to express TGR5, this raises the possibility that bile acids may also be stimulating the peripheral release of endogenous opioids.
The findings provide a direct molecular mechanism by which bile acids could contribute to the peripheral and central mechanisms of pruritus and altered analgesia associated with cholestasis. In addition, TGR5 has a distinct bile acid ligand specificity, which favors more hydrophobic species lacking hydroxylation at the 6, 7 or 12 positions.19 As such, it is certainly possible that the overall bile acid composition in the serum of patients includes both agonists and antagonists to TGR5, and thus may help explain patient-to-patient variable susceptibility to pruritus and poor correlation between total bile acid levels and pruritus severity. Finally, these results begin to “connect the dots” between bile acids, a liver-derived candidate pruritogen, and the changes in opioidergic signaling described in cholestatic liver disease.
The strengths of the study included use of state-of-the-art rodent models of itch and analgesia, and use of mouse models over-expressing or lacking TGR5. The study also had significant limitations. Most notable is the lack of human data or animal studies using a cholestatic model. The authors also use the unconjugated bile acid deoxycholic acid for many of their experiments, whereas this bile acid does not typically accumulate to high levels in cholestasis. Similar to the studies that identified LPA as a candidate pruritogen13, it will be critical to correlate pruritus severity with the TGR5 activity of the bile acid species present in serum of cholestatic patients. It is also important to note that other endogenous ligands besides bile acids have been identified for TGR5, such as progesterone, progesterone metabolites, and the neurosteroid pregnanolone among others.19 This observation raises the possibility that ligands other than or in addition to bile acids may be acting through TGR5 in cholestatic liver disease. This alternative hypothesis may be particularly relevant for Intrahepatic Cholestasis of Pregnancy, where elevated levels of progesterone metabolites have been implicated in the pathogenesis of the disease. Finally, it should be also noted that current findings do not challenge the concept of LPA as an important pruritogen in cholestatic liver disease. LPA is not a TGR5 agonist, but rather is believed to induce itch through other mechanisms, including mast cell degranulation4 and perhaps activation of specific LPA receptors on pruritoceptive neurons.13 Even as the present study has helped narrow important gaps in our understanding of the pathogenesis of cholestasis-associated itch (summarized in Figure 1), further investigation will certainly be required to determine if there is interaction between the LPA and TGR5-dependent neural pathways, and their individual roles at each stage in the progression of different forms of cholestatic liver disease.
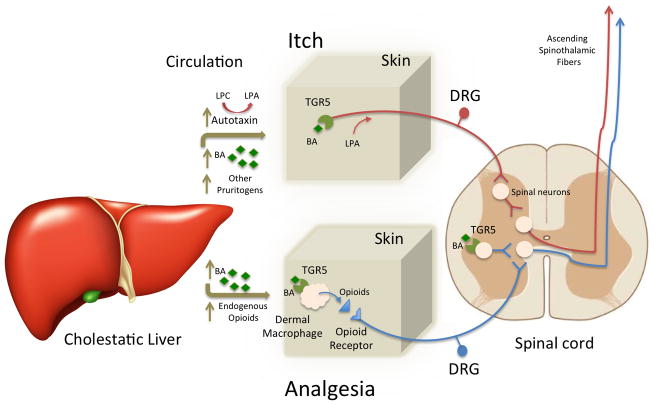
Fig 1.
Model for the pathogenesis of cholestatic liver disease-associated itch and altered analgesia. Cholestasis results in increased levels of circulating bile acids, autotaxin, and other potential pruritogens. Bile acids in skin may signal through their receptor TGR5 on peripheral sensory nerves to stimulate release of Gastrin-releasing peptide (GRP) and opioids by spinal neurons in the dorsal horn of the spinal cord. These neurotransmitters activate specific spinal neurons to induce itch. Autotaxin catalyzes the production of lysophosphatidic acid (LPA) from lysophosphatidylcholine (LPC), which can stimulate release of histamine or act directly on sensory nerves to induce itch. Bile acids could mediate analgesia by several mechanisms. In the skin, bile acids could activate TGR5 expressed on dermal macrophage to stimulate release of opioids. Bile acids may also activate TGR5 expressed on spinal neurons to stimulate release of opioids. BA, bile acids; DRG, dorsal root ganglion; LPA, lysophosphatidic acid; LPC, lysophosphatidylcholine.
What do these findings mean for our patients with cholestasis-associated itch? Our current armamentarium for relief of pruritus has its origins more in serendipity than mechanism and is clinically frustrating for all involved. Pruritus remains a debilitating component of cholestatic liver disease, and substantially impacts our patients’ well-being, sleep cycles, work performance, and may even lead to suicidal ideation. Thus, any novel insight into targets and mechanisms are welcome, and the work of Alemi and colleagues should help stimulate new, neural-based approaches. One can envision that this would be part of a systematic approach to therapy that integrates the type and stage of liver disease, genetic or microbiome influences, and serum levels of LPA/autotaxin and TGR5-potentiating agonists. The future should expand our understanding of this liver-bile acid-neuron axis and thus be a part of what can be offered to help our patients lead lives with one less “itch to scratch”.
Acknowledgments
This work was supported by NIH research grants DK047987 (P.A.D) and DK56239 (S.J.K.).
Abbreviations
- PFIC
progressive familial intrahepatic cholestasis
- LPA
lysophosphatidic acid
- LPC
lysophosphatidylcholine
- MARS
Molecular Adsorbents Recirculation System
- BA
bile acids
- DRG
dorsal root ganglion
- GRP
gastrin-releasing peptide
Footnotes
Conflict of Interest
Potential conflict of interest: Dr. Dawson has received funding from and consults for Lumena Pharmaceuticals. He has also consulted for GlaxoSmithKline. Dr. Karpen has no conflicts of interest.
References
- 1.Bergasa NV. The itch of liver disease. Seminars in cutaneous medicine and surgery. 2011;30:93–98. doi: 10.1016/j.sder.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Rishe E, Azarm A, Bergasa NV. Itch in primary biliary cirrhosis: a patients’ perspective. Acta dermato-venereologica. 2008;88:34–37. doi: 10.2340/00015555-0350. [DOI] [PubMed] [Google Scholar]
- 3.Kronsten V, Fitzpatrick E, Baker A. Management of Cholestatic Pruritus in Paediatric Patients With Alagille Syndrome: The King’s College Hospital Experience. Journal of pediatric gastroenterology and nutrition. 2013;57:149–154. doi: 10.1097/MPG.0b013e318297e384. [DOI] [PubMed] [Google Scholar]
- 4.Akiyama T, Carstens E. Neural processing of itch. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imam MH, Gossard AA, Sinakos E, Lindor KD. Pathogenesis and management of pruritus in cholestatic liver disease. Journal of gastroenterology and hepatology. 2012;27:1150–1158. doi: 10.1111/j.1440-1746.2012.07109.x. [DOI] [PubMed] [Google Scholar]
- 6.Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ. Primary biliary cirrhosis. Hepatology. 2009;50:291–308. doi: 10.1002/hep.22906. [DOI] [PubMed] [Google Scholar]
- 7.EASL Clinical Practice Guidelines: management of cholestatic liver diseases. Journal of hepatology. 2009;51:237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Varco RL. Intermittent external biliary drainage for relief of pruritus in certain chronic disorders of the liver. Surgery. 1947;21:43–45. [PubMed] [Google Scholar]
- 9.Datta DV, Sherlock S. Cholestyramine for long term relief of the pruritus complicating intrahepatic cholestasis. Gastroenterology. 1966;50:323–332. [PubMed] [Google Scholar]
- 10.Freedman MR, Holzbach RT, Ferguson DR. Pruritus in cholestasis: no direct causative role for bile acid retention. The American journal of medicine. 1981;70:1011–1016. doi: 10.1016/0002-9343(81)90857-3. [DOI] [PubMed] [Google Scholar]
- 11.Kuiper EM, van Erpecum KJ, Beuers U, Hansen BE, Thio HB, de Man RA, Janssen HL, et al. The potent bile acid sequestrant colesevelam is not effective in cholestatic pruritus: results of a double-blind, randomized, placebo-controlled trial. Hepatology. 2010;52:1334–1340. doi: 10.1002/hep.23821. [DOI] [PubMed] [Google Scholar]
- 12.Bergasa NV, Talbot TL, Alling DW, Schmitt JM, Walker EC, Baker BL, Korenman JC, et al. A controlled trial of naloxone infusions for the pruritus of chronic cholestasis. Gastroenterology. 1992;102:544–549. doi: 10.1016/0016-5085(92)90102-5. [DOI] [PubMed] [Google Scholar]
- 13.Kremer AE, Martens JJ, Kulik W, Rueff F, Kuiper EM, van Buuren HR, van Erpecum KJ, et al. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology. 2010;139:1008–1018. 1018 e1001. doi: 10.1053/j.gastro.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Kremer AE, van Dijk R, Leckie P, Schaap FG, Kuiper EM, Mettang T, Reiners KS, et al. Serum autotaxin is increased in pruritus of cholestasis, but not of other origin, and responds to therapeutic interventions. Hepatology. 2012;56:1391–1400. doi: 10.1002/hep.25748. [DOI] [PubMed] [Google Scholar]
- 15.Alemi F, Kwon E, Poole DP, Lieu T, Lyo V, Cattaruzza F, Cevikbas F, et al. The TGR5 receptor mediates bile acid-induced itch and analgesia. The Journal of clinical investigation. 2013;123:1513–1530. doi: 10.1172/JCI64551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Aguiar Vallim TQ, Tarling EJ, Edwards PA. Pleiotropic roles of bile acids in metabolism. Cell metabolism. 2013;17:657–669. doi: 10.1016/j.cmet.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alemi F, Poole DP, Chiu J, Schoonjans K, Cattaruzza F, Grider JR, Bunnett NW, et al. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology. 2013;144:145–154. doi: 10.1053/j.gastro.2012.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poole DP, Godfrey C, Cattaruzza F, Cottrell GS, Kirkland JG, Pelayo JC, Bunnett NW, et al. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil. 2010;22:814–825. e227–818. doi: 10.1111/j.1365-2982.2010.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato H, Macchiarulo A, Thomas C, Gioiello A, Une M, Hofmann AF, Saladin R, et al. Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure-activity relationships, and molecular modeling studies. J Med Chem. 2008;51:1831–1841. doi: 10.1021/jm7015864. [DOI] [PubMed] [Google Scholar]