Introduction
Pluripotent stem cells (PSCs), including embryonic (ESCs) and induced pluripotent stem cells (iPSCs), offer immense potential as a source for regenerative therapies, as was recently recognized by the 2012 Nobel Committee in Medicine. However, the intrinsic qualities of self-renewal and pluripotency that make these cells so therapeutically promising are also responsible for an equally fundamental tumorigenic potential. In this regard, PSC tumorigenicity can ultimately be divided into two separate categories: malignant transformation of differentiated PSCs and benign teratoma formation from residual undifferentiated PSCs, either of which can produce tumors consisting of either one or all three germ layers, respectively1.
The risks of PSC tumorigenicity have been highlighted over the past several years in a number of small and large animal studies, including preclinical dose-escalation tests for the first-in-human PSC clinical trial to be approved by the FDA in 2009. In this case, mice that received the Geron human ESC-derived neural progenitor cell (NPC) product GRNOPC1 developed cysts in regenerating tissue sites of the spine, prompting a one-year moratorium on the trial even before the first patient received treatment2. Other animal studies utilizing ESC- and iPSC-based therapies have shown further risk for PSC tumorigenic potential in humans. These include development of neural overgrowths and tumors from human ESC-derived dopaminergic neurons and NPCs transplanted into small animals, as well as ocular tumors in mice receiving ESC-derived retinal progenitors3–5. Moving one step further into primate models, human ESC-derived dopaminergic neurons transplanted into the brains of Parkinsonian monkeys have also resulted in tumors6. While PSC-derived tumors have yet to be reported in humans, several case studies have documented the formation of tumors in patients receiving fetal and adult stem cell treatments. These developments include the brain of a 12-year old boy who received fetal neural stem cell transplantation for treatment of ataxia telangiectasia7, and the kidney of a 46-year old woman who received autologous hematopoietic stem cell transplantation for treatment of lupus nephritis8.
The introduction of PSC derivatives to the clinic by the now defunct Geron-trial9, Advanced Cell Technology (ACT)10, and by the Center for Developmental Biology in Kobe11 risks the development of PSC-derived tumors in patients. On one side is the promise of a new era for regenerative medicine, and on the other is the risk of iatrogenic tumors, an occurrence that certainly slow progress in this field. In this perspective, we will discuss the hurdles to clinical implementation of PSCs associated with tumorigenicity and review current advances in addressing these challenges.
Conserved Gene Expression Networks Between Cancers and PSCs
Gene expression networks responsible for maintenance and induction of pluripotency in PSCs are interconnected and in many cases share components with those networks implicated in oncogenesis. Fundamental to both networks are genes that confer high proliferation capacity, self-renewal, DNA repair checkpoint uncoupling, and the ability to differentiate into multifaceted tissues. The central nature of such oncogenic properties is so fundamental to PSC identity that teratoma formation is a gold standard for demonstration of pluripotency in human PSCs. Of particular importance is the Myc transcription factor and the core pluripotency networks (i.e., Nanog, Oct4, and Sox2), which have emerged as fundamental gene circuits shared by PSCs and cancers12,13. Both of these transcriptional networks function to promote self-renewal, proliferation, and multipotency. However, activity of oncogenic transcription networks among PSCs should not be considered the sole domain of undifferentiated cells, as recent studies have demonstrated that differentiated cells may also retain or even re-activate such networks12,13. Narva et al. found that almost half of the genes (>44%) transcriptionally upregulated as a result of hESC genomic aberrations are functionally linked to cancer gene expression14. As a result, PSCs and their tumorigenic progeny exhibit cancer hallmarks, including in vitro lack of contact inhibition, loss of P53 and RB regulation of the cell cycle, and resistance to apoptosis1.
Although it is difficult to predict at what threshold of oncogenic gene activation results in tumor propagation, numerous groups have begun to qualitatively and quantitatively compare PSCs and cancers via large database-driven analyses. In this regard, Ben-Porath et al. described a correlation between more aggressive cancers and the expression of the core pluripotency and Myc-centered networks12. Conversely, expression of polycomb genes related to cell differentiation was associated with decreased cancer aggression. Kim et al. analyzed transcriptional networks by utilizing a stringent in vivo biotinylation technique to probe protein-protein and protein-DNA interactions13. This approach demonstrated a central role for the Myc transcriptional network in both cancers and PSCs. These analyses have provided insights into the identity and function of such gene networks and have elucidated their relationships to the global transcriptional networks necessary for pluripotency and oncogenesis. Moving forward, we believe that such bioinformatics screens should be viewed as hypothesis generating, prompting additional studies to elucidate functional consequences of the shared genetic expression profiles between cancers and PSCs. Due to the general lack of such studies, and even more fundamentally the lack of an objective scoring system to quantify these results, it is difficult to determine at what exact threshold expression of these genes becomes clinically concerning.
Considerable work has investigated the role of pluripotency gene networks in cancer. For example, Cui et al. found that the well-studied stem cell signaling pathway, Wnt, is a strong determinant for tumor formation from ESC-derived retinal progenitor cells5. A majority of work has also focused on Myc and its effectors15. The risks of the Myc oncogene are probably best highlighted in the case of iPSC derivation. To this end, reactivation of genomically integrated MYC in donor cells has been shown to produce somatic tumors in chimeric mice generated from iPSCs16,17. Although Myc has since been found to be dispensable for reprogramming18, the close association of this network with the fundamental core pluripotency factors suggests that inter-network crosstalk activates Myc or at least its effectors. The juxtaposition of these networks is further supported by a recent study by Lin et al. that demonstrated Myc-mediated tumorigenesis in PSCs through transcriptional amplification of oncogenic gene expression programs inherent to PSC identity19. These reports have established the oncogene Myc as a central player in oncogenesis and pluripotency, the ectopic activation of which is definitively linked to somatic tumor formation12,13.
In addition to the Myc network, other core pluripotency factors underlying ESC and iPSC identity have also been shown to promote cancer development. Ectopic activation of Oct4 in somatic cells, for example, induces dysplastic development and features of malignancy20. A number of studies have highlighted the inter-related nature of pluripotency and oncogenic gene networks in promoting adult somatic cancers. For example, Lee et al. demonstrated the role of Nanog in self-renewal of CD24+ cancer stem cells in hepatocellular carcinoma21. Sox2 has been shown to drive cancer cell survival and oncogenic fate in several cancer types, including squamous cell carcinomas of the lung and esophagus22. Klf-4 has been reported to promote DNA repair checkpoint uncoupling and cellular proliferation in breast cancers by P53 suppression23. Thus, it is apparent that transcription factors associated with pluripotency not only drive oncogenesis of PSC-derived teratomas, but also in clinical adult malignancies. This is evidenced in preclinical animal studies where inappropriate pluripotency gene expression in transplanted PSC derivatives results in single germ layer tumors such as proliferating neural rosettes and ocular tumors as opposed to teratoma formation3–6.
Aberrant activation of both Myc and Oct4 has also been noted in the case of “partially” reprogrammed colonies where inadequately silenced or re-activated genes result in a “pseudo-pluripotent” state exhibiting high levels of proliferation and resistance to differentiation24. It is difficult to assess the temporal sequence of events, e.g. whether Oct4 activates Myc or if the resultant tumorigenic cell properties are directly due to a cascade of more established oncogenic networks triggered by the initial ectopic expression of one or more pluripotency transgenes. As evidence mounts suggesting a role for the inappropriate regulation of Myc and Oct4 in PSC tumorigenesis, it is critical that the expression of core pluripotency factors and their relationships with established oncogenic networks are further studied to ensure patient safety.
Tumorigenicity associated with iPSC reprogramming
The recent derivation of iPSCs from somatic tissues by ectopic expression of core pluripotency factors adds additional concerns for PSC tumorigenicity (Table 1). As compared to ESC counterparts, iPSCs are exposed to a number of factors promoting oncogenic transformation, including genomic insertion of reprogramming vectors, overexpression of oncogenic transcription factors, and a global hypomethylation resembling that seen in cancers (Figure 1). Functional studies that directly compare iPSC and ESC tumorigenicity are currently lacking. However, numerous reports have highlighted the oncogenic risks associated with pluripotency induction1,18,19.
Table 1.
Oncogenic Risk Factors Resulting from Induction of Pluripotency
| 1. Integration of gene delivery vectors and transgenes into host cells |
| 2. Chromosomal damage during the reprogramming process |
| 3. Clonal selection for oncogenic or transformed colonies during PSC expansion |
| 4. Incomplete reprogramming |
| 5. Failure to silence pluripotency networks in differentiated progeny |
| 6. DNA damage accumulated during the cell culture or resulting from somatic mutations |
| 7. Aberrant regulation of the imprinting process |
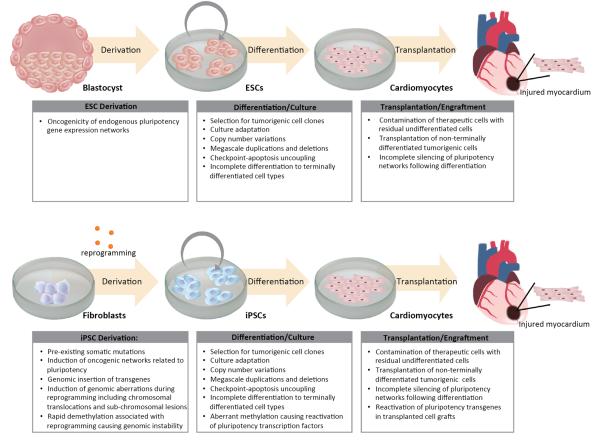
Figure 1. Tumorigenic hurdles to clinical translation of pluripotent stem cell based therapies.
Depicted are tumorigenic pitfalls associated with applications of pluripotent stem cells for patient treatment, using ischemic heart disease as an example. Specific areas of concern include derivation of ESCs/iPSCs, culture and differentiation into therapeutic cell populations (ie cardiomyocytes), delivery into sites of injury, and engraftment.
Strategies to diminish tumorigenic transformation of iPSCs have primarily focused on a variety of gene delivery vectors that minimize genomic disruption (Table 2). These methods can generally be divided into two categories: 1) integrating vectors that can be excised from the host genome and 2) non-integrating vectors. Proof-of-principle for derivation of iPSCs using excisable vectors was demonstrated by Soldner et al. who used a doxycycline inducible lentiviral construct flanked by loxP sites, allowing for excision by Cre-recombinase25. Because Cre-recombinase-driven excision leaves residual loxP sequences at sites of integration that could disrupt genomic coding or activate oncogenic promoters, Woltjen et al. followed this study by deriving iPSCs using integrating transposons that could subsequently be excised by piggyBac transposition26. Unlike Cre-recombinase, the use of transposon-based excision leaves no genomic trace and allows for xeno-free production of iPSCs. However, piggyBac transposition carries the risk of uncontrolled rounds of excision-integration, increasing the possibility of non-conservative deletions within coding regions.
Table 2.
Oncogenic Risks for Methods of Pluripotency Induction in Somatic Cells
| Method of Induction | Strengths | Weaknesses |
|---|---|---|
| Lentiviral vector | Robust reprogramming efficiency | Genomic integration, Reactivation of integrated transgenes |
| Adenoviral vector | Low risk for genomic integration | Low transduction efficiency, Limited transgene expression |
| Cre recombinase | Little genomic disruption | Low transduction efficiency, Integration of LoxP sites into host genome |
| PiggyBac transposition | Minimal risk for genomic disruption | Low transduction efficiency, Risk for uncontrolled rounds of excision-integration |
| Plasmid transduction | Minimal risk for genomic disruption | Very low transduction efficiency, Typically require use of oncogenes such as SV40LT antigen for successful induction of pluripotency |
| Minicircle | Minimal risk for genomic disruption | Low transduction efficiency |
| Sendai Virus | Minimal risk for genomic integration, relatively high transduction efficiency | Risk for continuous replication of viral vector in cytoplasm leading to improper silencing of pluripotency transgenes |
| Synthetic mRNA | No risk for genomic integration, ability to control transgene expression | Variable transduction efficiencies, High technical expertise required |
| Protein transduction | No risk for genomic integration, ability to control transgene expression | Very low transduction efficiency, Labor intensive |
| Small molecules | No risk for genomic integration | Variable off-target effects |
Non-integrating techniques such as epichromosomal viruses and plasmids have also been investigated as a means to circumvent integration and reactivation of potentially oncogenic reprogramming factors. These methodologies are generally considered safer than excisable integration techniques as they avoid even temporary genomic modifications. However, standard non-integrating strategies such as adenovirus suffer from extremely low transduction efficiencies (0.001%), and in the case of episomal plasmids, require the use of potent oncogenes such as the SV40LT antigen for pluripotency induction27,28. Recent efforts have thus focused on the identification of novel and more efficient vectors such as the Sendai virus29, a RNA virus lacking a DNA phase and therefore exhibit zero risk of genomic integration. The major disadvantage to this approach is that this virus is known to continuously replicate in the cytoplasm producing reprogramming transgenes that may avoid adequate silencing.
A number of studies have reprogrammed somatic cells by direct delivery of synthetic mRNAs and pluripotency proteins30,31. The primary advantages of these techniques are the virtual elimination of integration risk and the ability to engineer controlled transgene expression. However, reprogramming efficiency is difficult to maintain, requiring daily transfections for prolonged periods of time. In addition, the required technical skill is considerable and only a handful of centers have been able to successfully apply these strategies.
Finally, several recent studies have reported the successful elimination of Myc from reprogramming cocktails18. These strategies improve the safety of iPSC generation at the expense of efficiency. Substitution of c-Myc with L-Myc has been demonstrated as a viable method to retain higher reprogramming efficiencies without the risk of Myc-induced oncogenesis32. Another strategy to avoid the utilization of Myc or other transcription factors while maintaining reprogramming efficiency is the use of small molecules33. However, small molecules have off-target effects capable of inducing oncogenesis, many of which have yet to be fully elucidated. Until the ramifications of these compounds are better understood, the clinical applicability of this approach is unclear.
The induction of pluripotency itself has also been linked to tumorigenic transformation by creating genomic aberrations at chromosomal and sub-chromosomal levels (Figure 2). Laurent et al. conducted the first study to compare high-resolution single nucleotide polymorphism (SNP) profiles of ESC, iPSC, somatic stem cell, and primary adult cell lines34. Importantly, the authors found numerous deletions of tumor-suppressor genes in iPSCs immediately after pluripotency induction that were absent from the somatic cells of origin. Hussein et al. reported similar findings in utilizing high-resolution SNP arrays to compare copy number variations in early and late passage iPSCs with their somatic cells of origin. The authors suggested replication stress associated with the reprogramming process was responsible for de novo mutations arising during pluripotency induction, indicating the process of demethylation itself results in genomic structural instability35. Another potential mechanism for how demethylation could produce such lesions is by facilitating transcriptional access to proto-oncogenes for cancer formation.
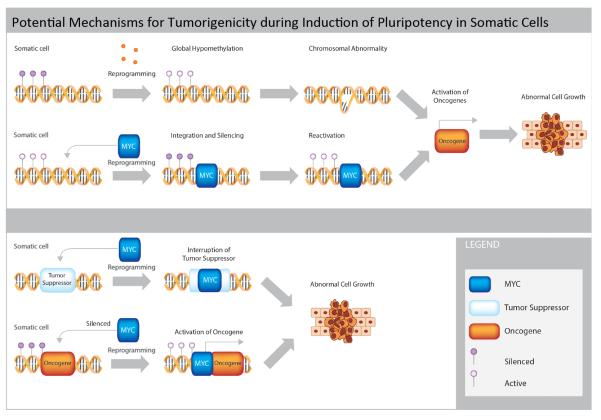
Figure 2. Potential mechanisms for tumorigenicity during induction of pluripotency in somatic cells.
This figure illustrates the common mechanisms by which the process of reprogramming somatic cells to pluripotency may directly result in promotion of tumorigenic outcomes. Specifically, global epigenetic hypomethylation (purple circles) may directly cause chromosomal abnormalities and oncogene activation. Integration of pluripotency transgenes such as MYC (blue) may result in reactivation of oncogenic networks following cell differentiation and transplantation into patients. Genomic integration of transgenes may also directly inactivate tumor suppressors (white) or activate oncogenes (orange), causing abnormal cell growth.
Global hypomethylation has been shown to exist in both PSCs and cancers36. Chromosome-remodeling factors that facilitate reprograming through demethylation have been associated with somatic tumors and cancer stem cells through activation of previously silenced oncogenes37. Aberrant methylation has also been linked to insufficient inactivation or reactivation of pluripotency transgenes, leading to tumor development via induction of genomic lesions secondary to hasty and error-prone replication36. Several reports have also demonstrated genomic hypomethylation alone is sufficient to induce chromosomal translocations resulting in tumorigenesis. Seminal work in this area has been by conducted by the Jaenisch lab, showing that induction of a hypomorphic allele DNA methyltransferase 1 (DNMT1) allele results in genomes with only 10% methylation which facilitates loss of heterozygosis in oncogenic neurofibromatosis and p53 genes38. Such lesions may be due to the fact that methylation offers a level of structural genetic stability and that its marked removal therefore promotes genomic instability. As induction of pluripotency produces global hypomethylation, it is necessary to understand the respective oncogenic contributions of the reprogramming process itself compared with other potential causes of PSC tumorigenicity such as culture adaptation.
Impact of Culture Adaptation upon ESCs and iPSCs
In addition to the tumorigenic risks inherent to pluripotency induction, culture adaptation is another prevalent mechanism known to activate oncogenic networks. In this regard, current models of cell therapy can require hundreds of millions to billions of cells per patient39. The development of scalable cell culture methods to achieve these numbers is a high priority and will likely require significant periods of in vitro expansion. It is important to note that the PSC state is analogous to the inner cell mass, a highly transitory stage of development not meant for indefinite propagation. It is therefore not surprising that chromosome instability is naturally prevalent in early embryos, a fact that becomes apparent when embryos are artificially selected and implanted during in vitro fertilization40. In addition, several recent studies have demonstrated that developmentally immature PSCs exhibit deficient DNA damage repair and cell cycle arrest41. While PSCs may respond to DNA damage and replicative stress through apoptosis, a minority of cells do not and continue to proliferate even after sustaining genetic lesions42. As a result, prolonged PSC culture produces genomic abnormalities, including chromosomal aneuploidy, translocations, mega-scale duplications/deletions, and point mutations43. Although the generation of such genetic lesions is stochastic, their accumulation is not. Subclones that gain selective growth advantages either due to loss of tumor suppressor genes or gain of proliferation genes will out-compete others in vitro. The net result is the propagation of cultures that have steadily and heterogeneously acquired tumorigenic potential.
The effect of culture adaption is most noticeable in the accumulation of gross chromosomal abnormalities in high passage PSCs. Draper et al. were the first to specifically characterize such genomic lesions and showed that prolonged ESC culture produces recurrent gain of chromosomes 17q and 1244. This work has been followed by others who continually expand the list of recurrent genetic lesions45,46. Taking this work one step further, Mayshar et al. compared the genomic integrity of human ESCs and iPSCs to demonstrate that many of the genetic abnormalities found in ESCs and germ cell cancers are duplicated in iPSCs due to culture adaptation43. Notably, these changes include copy number gains in chromosome 12p, which contains the pluripotency transcription factor Nanog and numerous growth and survival genes such as Stellar and GDF3. Expanding this analysis to 38 international laboratories, the International Stem Cell Initiative (ISCI) recently completed the largest surveys of PSC genetic variability, comparing 125 human ESC and 11 iPSC lines47. This study found karyotype abnormalities in 34% of lines tested, with more than 20% harboring a conserved 20q11.12 mutation that conferred increased activity of the anti-apoptotic gene Bcl2L147. Ultimately, these genetic modifications have profound functional implications, promoting tumorigenic qualities such as increased proliferation, growth factor independence, niche autonomy, and higher frequencies of tumor-initiating cells48.
The fact that such genetic lesions are generated stochastically suggests that significant variations can arise between labs, even between subclones from the same cell lines. Indeed, comparisons of identical cell lines grown in different laboratories demonstrates that disparate culture conditions can result in long-term, lab-specific gene expression profiles49. These results highlight the impact of culture conditions on PSC gene expression. An explanation for such variations can be inferred from work by Narsinh et al. that demonstrate significant cell-to-cell variability within the same PSC colony, implying significant plasticity in the pluripotent state50. These findings highlight the need for stringent enforcement of in vitro culture and maintenance standards across PSC types, lines, and lab-specific subclones to ensure patient safety.
Teratomas, immunogenicity, and tumor removal strategies
The potential of PSCs and their differentiated progeny to form tumors upon transplantation offers one of largest hurdles for clinical entry. To this end, one of the major factors influencing oncogenic development is immune recognition of both differentiated and undifferentiated PSCs. On one hand, a high level of immune function would prevent tumor formation to the extent that the entire graft would be rejected. On the other hand, reduced immunogenicity would facilitate cell engraftment but also allow for tumor formation in the event of donor cell misbehavior. In this regard, two categories of PSC grafts exist with disparate immunogenic properties: autologous and allogeneic cell transplantations.
One of the primary appeals of iPSC development is the potential to create autologous grafts that evade immune rejection. While significant controversy exists within this field51, current evidence supports the hypothesis that autologous iPSC derived grafts are not strongly immunogenic. Recent work by Guha et al. and Araki et al. utilized syngeneic mouse models to demonstrate that transplanted iPSC-derived embryoid bodies, skin, and bone marrow tissues are efficiently engraft with almost no signs of rejection52,53. Such immune evasion is desirable as it obviates the need for the harsh immunosuppression regimes required by conventional allogeneic transplant strategies. However, it is important to note that immune privileged status may be potentially shared by aberrant cells existing within grafts, and could facilitate the development of tumors derived from host tissue.
While iPSCs may reach the clinic as early as late 201311, clinical trials utilizing ESC derivatives began in earnest from 20109,10. ESC-derived grafts are fundamentally different from iPSC-derived grafts in that ESCs are derived from the excess blastocysts discarded by IVF clinics and hence can only be transplanted allogeneically. Drukker et al. were the first to characterize ESC immunogenicity and showed decreased MHC expression in undifferentiated PSCs as compared to their differentiated progeny54. Such a trend is problematic as undifferentiated cells would be less susceptible to immune recognition as compared to therapeutic cell types derived from these cells. Further complicating this picture is the interplay of other cellular immune effectors outside of T-cells, namely NK cells, which recognize cells that exhibit decreased MHC expression55. As such, further study into the complex interplay of the immune system must be undertaken before we can fully understand PSC immunogenicity. However, it is likely that because differentiation results in increased immune recognition marker expression, any allogeneic PSC-derived graft would likely require some degree of immune suppression56,57. Therefore a balance between tumorigencity and immunogenicity must be achieved. Such a balance will likely require a tailored approach depending on the patient population, delivery site, and/or therapeutic product58.
Prospective removal (e.g., removal before transplantation) of tumorigenic cells is preferable to retrospective cancer treatments, as prospective methods would provide the highest level of patient safety while reducing the need for post-transplantation surveillance. Prospective removal is optimally achieved through utilizing intrinsic cell properties such as surface antigens. These techniques include antibody-induced cytotoxicity59 and fluorescent activated cell sorting (FACS)/magnetic activated cell sorting (MACS) depletion based on pluripotency-associated surface markers60 or enrichment based on surface markers associated with differentiation61. The key to any such technique is the identification or insertion of markers or cellular traits that change drastically upon PSC differentiation. Such a trait is highlighted by a recent paper by Ben-David et al., which identified a novel metabolic pathway inherent to undifferentiated cell survival. By developing a small molecular inhibitor of stearoyl-coA desaturase, a key enzyme within this pathway, the authors were able to selectively eliminate undifferentiated PSCs from culture62. Although these strategies are promising for teratoma prevention, further studies must be conducted to ensure that the proposed methods of PSC depletion do not inadvertently hinder engraftment of therapeutic cell types or more subtly interfere with graft integration, function, or long-term survival. Ideally, the most stringent safety regimes would utilize a flexible, combinatorial approach that may require tailoring for specific PSC lines or graft types. Should these techniques fail to adequately remove enough residual PSCs, retrospective tumor treatments may also be employed, including oncologic chemotherapy, radiation, and surgery63 or the incorporation of suicide ablation genes64.
In addition to removing residual undifferentiated cells, attention should also be given to ensure the removal of genetically abnormal cells, independent of their differentiation status. Although little work has been done in this area, Herszfeld et al. provided the first evidence that CD30 expression is correlated with genetic abnormalities65. Such a cell surface target provides a convenient marker for FACS or MACS based separation and highlights the fact that we must first fully understand the most common and or most clinically relevant genetic lesions before we can confidently ensure removal of genetically aberrant PSCs and their derivatives. Ultimately, the risks of tumor formation from undifferentiated PSCs should be considered a significant but surmountable hurdle that requires careful study of PSC biology so that we can develop the necessary stringent manufacturing practices and high levels of quality control.
Conclusion
More than therapeutic efficacy, risks to patient safety are a primary focus in phase 1 clinical trials that often dictate the opinions of both the scientific community and lay public. In this regard, tumorigenicity is a potential hurdle that could halt ESC and iPSC research. It is therefore imperative that researchers thoroughly and systematically investigate the factors influencing PSC tumor formation before proceeding with large-scale clinical implementation. It is important to note that although we describe a number of significant contributors to tumorigenicity, their relative importance in producing a clinical phenotype is unknown and requires further investigation. Preclinical studies conducted by researchers in industry and academia have demonstrated tumor formation from PSC-derived therapies is a distinct possibility, with the reported developments of cystic structures, primitive tissue grafts and teratomas in animal models3–6.
Looking ahead, the next major test for PSC safety lies in the results of the first-in-human PSC-based clinical trials initiated by Geron9, ACT10, and the Kobe Center for Developmental Biology11. To date, these trials have enrolled a total of 15 known patients. Five of these patients received Geron's oligodendrocyte progenitor cell therapy (GRNOPC1) in the setting of acute spinal cord injury prior to the closure of this trial. An additional 10 patients received PSC-derived retinal pigment epithelial cells for Stargardt's macular dystrophy as part of the first phase of ACT's Phase 1/2 trial. With regards to ACT, preliminary reports on 2 patients have been reported by Schwartz et al. who noted no tumor formation or signs of rejection within the first four months of transplantation10. Moving forward, plans are underway to begin the first iPSC clinical trial in Japan, which aims to treat 6 patients with age-related macular degeneration using iPSC-derived retinal epithelial cells11. Caveats to these first PSC trials are that the cell numbers employed are several orders of magnitude lower than adult stem cell clinical trials, which typically use hundreds of millions to billions of cells. In addition, tests to monitor for graft survival are limited, so the absence of tumor formation in patients may potentially be attributed to failure of donor cells to engraft. As more human trials are approved and patient data is generated, our understanding of the tumorigenic potential of PSC-based therapies will continue to evolve.
As our knowledge of PSC biology develops, especially with improved methods to generate these cells and their differentiated progeny, it is likely that further factors influencing tumorigenicity will emerge. Because PSCs are derived and maintained using artificial techniques that, the biology of these cells deviates substantially from their natural in vivo environment, the inner cell mass. It is therefore not surprising that troubling oncologic lesions and phenotypes develop with our often crude manipulations. Our duty as PSC biologists and clinicians should be to thoroughly understand these cells of our own creation before we can move PSC-based therapies to the patient bedside.
Acknowledgements
We thank Amy Morris and June Wha-Rhee for preparing the illustrations. Due to space constraints, we are unable to include all relevant studies regarding the tumorigenicity of pluripotent stem cells; we apologize to those investigators whose valuable work we have omitted. This work was in part supported by National Institutes of Health Grants HL093172, HL099117, EB009689, and Burroughs Wellcome Fund Career Award in the Biomedical Sciences (J.C.W.); by California Institute of Regenerative Medicine Tools & Technology II (I.L.W.); and by Howard Hughes Medical Institute research training fellowships (A.S.L. and C.T.).
References
- 1.Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nature Reviews Cancer. 2011;11:268–277. doi: 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- 2.DeFrancesco L. Fits and starts for Geron. Nature Biotechnology. 2009;27:877. [Google Scholar]
- 3.Roy NS, et al. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nature Medicine. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- 4.Kriks S, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui L, et al. WNT signaling determines tumorigenicity and function of ESC-derived retinal progenitors. The Journal of Clinical Investigation. 2013;123:1647–1661. doi: 10.1172/JCI65048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doi D, et al. Prolonged maturation culture favors a reduction in the tumorigenicity and the dopaminergic function of human ESC-derived neural cells in a primate model of Parkinson's disease. Stem Cells. 2012;30:935–945. doi: 10.1002/stem.1060. [DOI] [PubMed] [Google Scholar]
- 7.Amariglio N, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Medicine. 2009;6:e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cyranoski D. Strange lesions after stem-cell therapy. Nature. 2010;465:997. doi: 10.1038/465997a. [DOI] [PubMed] [Google Scholar]
- 9.Strauss S. Geron trial resumes, but standards for stem cell trials remain elusive. Nature Biotechnology. 2010;28:989–990. doi: 10.1038/nbt1010-989. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz SD, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 11.Cyranoski D. Stem cells cruise to clinic. Nature. 2013;494:413. doi: 10.1038/494413a. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Porath I, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nature Genetics. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J, et al. A Myc network accounts for similarities between embryonic stem and cancer cell transcription programs. Cell. 2010;143:313–324. doi: 10.1016/j.cell.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narva E, et al. High-resolution DNA analysis of human embryonic stem cell lines reveals culture-induced copy number changes and loss of heterozygosity. Nature Biotechnology. 2010;28:371–377. doi: 10.1038/nbt.1615. [DOI] [PubMed] [Google Scholar]
- 15.Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nature Reviews Cancer. 2008;8:976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 16.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 17.Markoulaki S, et al. Transgenic mice with defined combinations of drug-inducible reprogramming factors. Nature Biotechnology. 2009;27:169–171. doi: 10.1038/nbt.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wernig M, Meissner A, Cassady JP, Jaenisch R. c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell. 2008;2:10–12. doi: 10.1016/j.stem.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Lin CY, et al. Transcriptional amplification in tumor cells with elevated c-Myc. Cell. 2012;151:56–67. doi: 10.1016/j.cell.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121:465–477. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 21.Lee TK, Cheung VC, Ng IO. Liver tumor-initiating cells as a therapeutic target for hepatocellular carcinoma. Cancer Letters. 2012 doi: 10.1016/j.canlet.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Bass AJ, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nature Genetics. 2009;41:1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rowland BD, Bernards R, Peeper DS. The KLF4 tumour suppressor is a transcriptional repressor of p53 that acts as a context-dependent oncogene. Nature Cell Biology. 2005;7:1074–1082. doi: 10.1038/ncb1314. [DOI] [PubMed] [Google Scholar]
- 24.Mikkelsen TS, et al. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008;454:49–55. doi: 10.1038/nature07056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soldner F, et al. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woltjen K, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu J, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ban H, et al. Efficient generation of transgene-free human induced pluripotent stem cells (iPSCs) by temperature-sensitive Sendai virus vectors. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14234–14239. doi: 10.1073/pnas.1103509108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warren L, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J, et al. Activation of innate immunity is required for efficient nuclear reprogramming. Cell. 2012;151:547–558. doi: 10.1016/j.cell.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakagawa M, Takizawa N, Narita M, Ichisaka T, Yamanaka S. Promotion of direct reprogramming by transformation-deficient Myc. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14152–14157. doi: 10.1073/pnas.1009374107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desponts C, Ding S. Using small molecules to improve generation of induced pluripotent stem cells from somatic cells. Methods in Molecular Biology. 2010;636:207–218. doi: 10.1007/978-1-60761-691-7_13. [DOI] [PubMed] [Google Scholar]
- 34.Laurent LC, et al. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell. 2011;8:106–118. doi: 10.1016/j.stem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hussein SM, et al. Copy number variation and selection during reprogramming to pluripotency. Nature. 2011;471:58–62. doi: 10.1038/nature09871. [DOI] [PubMed] [Google Scholar]
- 36.Ohm JE, et al. Cancer-related epigenome changes associated with reprogramming to induced pluripotent stem cells. Cancer Research. 2010;70:7662–7673. doi: 10.1158/0008-5472.CAN-10-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris WJ, et al. The histone demethylase KDM1A sustains the oncogenic potential of MLL-AF9 leukemia stem cells. Cancer Cell. 2012;21:473–487. doi: 10.1016/j.ccr.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 38.Gaudet F, et al. Induction of tumors in mice by genomic hypomethylation. Science. 2003;300:489–492. doi: 10.1126/science.1083558. [DOI] [PubMed] [Google Scholar]
- 39.Wollert KC, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 40.Vanneste E, et al. Chromosome instability is common in human cleavage-stage embryos. Nature Medicine. 2009;15:577–583. doi: 10.1038/nm.1924. [DOI] [PubMed] [Google Scholar]
- 41.Hyka-Nouspikel N, et al. Deficient DNA damage response and cell cycle checkpoints lead to accumulation of point mutations in human embryonic stem cells. Stem Cells. 2012;30:1901–1910. doi: 10.1002/stem.1177. [DOI] [PubMed] [Google Scholar]
- 42.Desmarais JA, et al. Human embryonic stem cells fail to activate CHK1 and commit to apoptosis in response to DNA replication stress. Stem Cells. 2012;30:1385–1393. doi: 10.1002/stem.1117. [DOI] [PubMed] [Google Scholar]
- 43.Mayshar Y, et al. Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell Stem Cell. 2010;7:521–531. doi: 10.1016/j.stem.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 44.Draper JS, et al. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nature Biotechnology. 2004;22:53–54. doi: 10.1038/nbt922. [DOI] [PubMed] [Google Scholar]
- 45.Lefort N, et al. Human embryonic stem cells reveal recurrent genomic instability at 20q11.21. Nature Biotechnology. 2008;26:1364–1366. doi: 10.1038/nbt.1509. [DOI] [PubMed] [Google Scholar]
- 46.Spits C, et al. Recurrent chromosomal abnormalities in human embryonic stem cells. Nature Biotechnology. 2008;26:1361–1363. doi: 10.1038/nbt.1510. [DOI] [PubMed] [Google Scholar]
- 47.Amps K, et al. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nature Biotechnology. 2011 doi: 10.1038/nbt.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Werbowetski-Ogilvie TE, et al. Characterization of human embryonic stem cells with features of neoplastic progression. Nat Biotechnol. 2009;27:91–97. doi: 10.1038/nbt.1516. [DOI] [PubMed] [Google Scholar]
- 49.Newman AM, Cooper JB. Lab-specific gene expression signatures in pluripotent stem cells. Cell Stem Cell. 2010;7:258–262. doi: 10.1016/j.stem.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 50.Narsinh KH, et al. Single cell transcriptional profiling reveals heterogeneity of human induced pluripotent stem cells. The Journal of Clinical Investigation. 2011;121:1217–1221. doi: 10.1172/JCI44635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 52.Araki R, et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494:100–104. doi: 10.1038/nature11807. [DOI] [PubMed] [Google Scholar]
- 53.Guha P, Morgan JW, Mostoslavsky G, Rodrigues NP, Boyd AS. Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell Stem Cell. 2013;12:407–412. doi: 10.1016/j.stem.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 54.Drukker M, et al. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99:9864–9869. doi: 10.1073/pnas.142298299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rideout WM, 3rd, Hochedlinger K, Kyba M, Daley GQ, Jaenisch R. Correction of a genetic defect by nuclear transplantation and combined cell and gene therapy. Cell. 2002;109:17–27. doi: 10.1016/s0092-8674(02)00681-5. [DOI] [PubMed] [Google Scholar]
- 56.Pearl JI, et al. Short-term immunosuppression promotes engraftment of embryonic and induced pluripotent stem cells. Cell Stem Cell. 2011;8:309–317. doi: 10.1016/j.stem.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swijnenburg RJ, et al. Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12991–12996. doi: 10.1073/pnas.0805802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwartz SD, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012 doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 59.Choo AB, et al. Selection against undifferentiated human embryonic stem cells by a cytotoxic antibody recognizing podocalyxin-like protein-1. Stem Cells. 2008;26:1454–1463. doi: 10.1634/stemcells.2007-0576. [DOI] [PubMed] [Google Scholar]
- 60.Tang C, et al. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nature Biotechnology. 2011;29:829–834. doi: 10.1038/nbt.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dubois NC, et al. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nature Biotechnol. 2011;29:1011–1018. doi: 10.1038/nbt.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ben-David U, et al. Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell. 2013;12:167–179. doi: 10.1016/j.stem.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 63.Peckham MJ, McElwain TJ, Barrett A, Hendry WF. Combined management of malignant teratoma of the testis. Lancet. 1979;2:267–270. doi: 10.1016/s0140-6736(79)90288-5. [DOI] [PubMed] [Google Scholar]
- 64.Di Stasi A, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. New England Journal of Medicine. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herszfeld D, et al. CD30 is a survival factor and a biomarker for transformed human pluripotent stem cells. Nature Biotechnology. 2006;24:351–357. doi: 10.1038/nbt1197. [DOI] [PubMed] [Google Scholar]