Figure 2.
Interactions of Arabidopsis ATG11 with Itself, Subunits of the ATG1/13 Kinase Complex, and ATG8-Decorated Autophagic Structures.
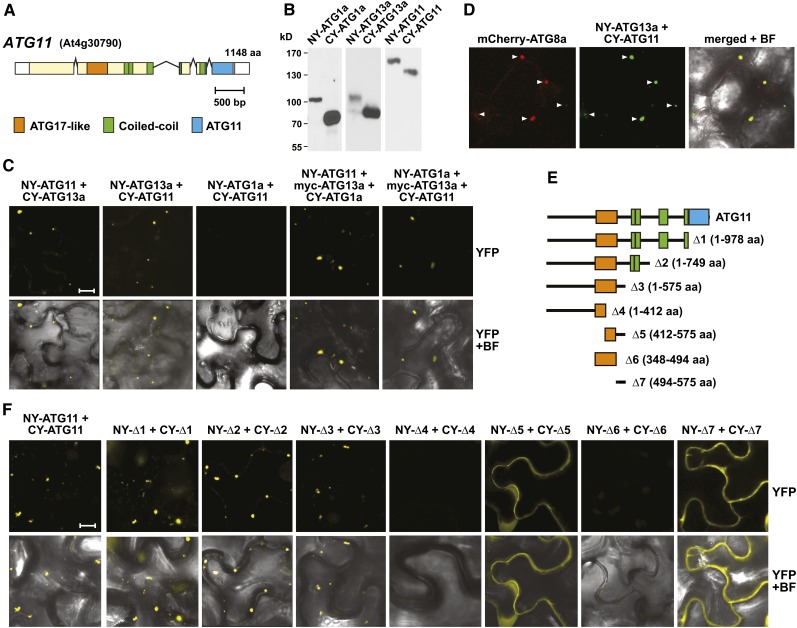
(A) Domain organization of ATG11. Lines represent introns, and colored and white boxes represent coding and untranslated regions, respectively. Positions of the signature ATG17-like, coiled-coil, and ATG11 domains are indicated. aa, amino acids.
(B) and (C) Interaction of ATG11 with ATG1 and ATG13 in planta by BiFC.
(B) Demonstration that each possible orientation of the NY and CY constructions for ATG1a, ATG11, and ATG13a could be expressed in N. benthamiana. Crude extracts prepared from leaves 36 h after infiltration were immunoblotted with anti-GFP antibodies.
(C) BiFC using N. benthamiana leaf epidermal cells. Leaves were coinfiltrated with plasmids expressing the N- and C-terminal fragments of YFP fused to ATG1a, ATG11, and ATG13a. To detect indirect interactions between ATG1a and ATG11, the cells were also infiltrated with a plasmid expressing ATG13a tagged with an N-terminal Myc epitope. Shown are reconstituted BiFC signals as detected by confocal fluorescence microscopy of leaf epidermal cells 36 h after coinfiltration along with a bright-field (BF) image of the cells. Bar = 10 μm.
(D) ATG11 and ATG13a colocalize with ATG8-decorated autophagic structures. The leaves of 4-d-old transgenic Arabidopsis seedlings stably expressing mCherry-ATG8a were coinfiltrated with plasmids expressing the NY-ATG13a and CY-ATG11 BiFC constructs and examined by confocal fluorescence microscopy as in (C). Arrowheads indicate colocalized structures.
(E) and (F) Localization of the homodimerization domain within ATG11 by BiFC.
(E) Diagram of the ATG11 truncations.
(F) BiFC signals in N. benthamiana leaf epidermal cells from paired ATG11 truncations fused to the N- and C-terminal fragments of YFP along with a bright-field image of the cells. Bar = 10 μm.