Abstract
This perspective article proposes a conceptual model for the pain experience for individuals diagnosed with knee osteoarthritis (OA). Pain in knee OA is likely a heterogeneous, multifactorial phenomenon that involves not only the OA disease process but also elements specific to patient psychology and pain neurophysiology. The relevant contributions to the pain experience for any individual patient remain difficult, if not impossible, to definitively determine, and the rationale for many clinical treatment decisions arises primarily from a mechanistic understanding of OA pathophysiology. The Osteoarthritis Research Society International (OARSI) recently identified “phenotyping” of OA pain as a research priority to “better target pain therapies to individual patients.” This perspective article proposes that contributions from 3 domains—knee pathology, psychological distress, and pain neurophysiology—should be considered equally important in future efforts to understand pain phenotypes in knee OA. Ultimately, characterization of pain phenotypes may aid in the understanding of the pain experience and the development of interventions specific to pain for individual patients.
The diagnosis “knee osteoarthritis (OA)” has evolved over the years into a description of complex and varied mechanical, biochemical, and structural processes occurring primarily at the knee joint. However, the hallmark of knee OA that typically drives clinical decision making is the patient's complaint of pain. The link between knee pain and signs of knee pathology that are characteristic of OA has been described as tenuous, and the tendency to attribute certain kinds of knee pain to OA has led to a heterogeneous clinical population for whom interventions often are ineffective at diminishing pain.1–3 A similar heterogeneity exists across many chronic musculoskeletal pain conditions. Research efforts in other populations—including low back pain, fibromyalgia, and temporomandibular disorder—have attempted to reduce heterogeneity by defining subgroups of people who have a similar pain phenotype, with the idea that interventions might then be directed more specifically at an individual's pain experience. Similar pain phenotyping efforts may be important for advancing treatment strategies in knee OA, although our understanding of knee OA subgroups is now in its infancy. In this perspective article, we propose a model for pain in knee OA involving a confluence of peripheral (knee pathology) factors, psychological factors, and neurophysiological (central pain processing) factors. Based on this model, future research may be able to define the relevant variables or combinations of variables that give rise to the clinical pain experience for individuals with knee OA.
Knee OA: What's in a Name?
Osteoarthritis is one of the most common diagnoses in primary care; arthropathies, in general, rank third, behind essential hypertension and acute respiratory infection, in reasons for adult ambulatory care visits in the United States.4 The label “knee OA” is applied to approximately 12% of older adults or 4.3 million Americans.5 It has been linked to diminished physical function, poor quality of life, and reduced life expectancy.6,7 However, knee OA is also a diagnosis known to encompass patients across a wide range of ages, with different levels of physical function and diverse priorities in terms of recreational activities.8 Clinical criteria for the diagnosis of OA are subject to interpretation and often used inconsistently in practice.9 Furthermore, although knee OA is considered a degenerative condition, progression is highly variable; many people do not exhibit signs of worsening symptoms or advancing joint degeneration, even over the course of several years.10,11 In short, the label “knee OA” does not appear to provide much information regarding the prognosis of individual patients, and the clinical utility of knee OA as a diagnosis has been called into question.12
Historically, the term “osteoarthritis” was intended to describe a common clinical observation: the troublingly painful and deformed joint, emblematic of disability for many older adults. Efforts to understand the pathophysiology of this condition led to the radiographic characterization of articular cartilage degradation and subchondral bone sclerosis,13 which subsequently came to define OA in the minds of many clinicians. Even today, radiographic signs of OA—including joint space narrowing and osteophyte formation—are heavily relied upon in clinical practice and widely regarded by physicians to be the gold standard for diagnosis.14 However, the causal relationship between radiographic OA and its primary clinical signature (ie, pain) remains poorly understood. In large-scale studies of older adults with knee pain, only about half of the people display evidence of radiographic OA.15,16 Conversely, of all people with radiographic knee OA, only about half report pain.16
This discrepancy has prompted the speculation that radiographic signs of knee OA, at least as classically defined, might not represent disease but might occur as a relatively innocuous part of the aging process,17,18 perhaps explaining why some people with severe knee degeneration do not report concurrent knee pain. Alternatively, other authors have suggested that radiographic diagnostic methods lack sensitivity to detect the “active” pathology relevant to pain,19–22 thus explaining why some people with minimal degeneration report high levels of pain. Recent evidence suggests that a relationship exists between severity of knee OA and pain; people with more severe radiographic evidence OA are indeed more likely to report pain,16,23 and people with discrepant knees (ie, experiencing more frequent knee pain in one knee compared with the other) appear particularly likely to report more pain in a knee if it displays more signs of radiographic degeneration.15 However, in population studies where multiple radiographic views have been used to maximize the sensitivity to pain, the specificity of the diagnosis appears to markedly diminish (ie, many people who are asymptomatic are identified as having knee OA).24,25 Thus, the isolated use of knee radiography appears to provide limited information regarding the experience of knee pain.
Perhaps because of the limitations inherent to the diagnostic capabilities of radiography, various factors that could additionally contribute to the pathophysiology of OA have been extensively studied, and a definition of OA that assimilates this knowledge has been proposed.26 This definition encompasses structures such as articular cartilage, subchondral bone, ligaments, joint capsule, synovial membrane, and periarticular muscles, and it involves a complex of hereditary, biochemical, and mechanical processes. Unfortunately, this expanded definition may now be so broad as to be largely unhelpful in clarifying OA disease pathogenesis.27 Furthermore, it fails to balance efforts to better understand OA pathophysiology with efforts to meet the clinical obligation to improve patients' pain, function, and social participation. Thus, a 2000 consensus meeting for the National Institutes of Health (NIH) concluded that “it is important to separate conceptually the disease process of OA and the syndrome of musculoskeletal pain and disability.”12(p642)
For clinicians, armed with interventions primarily directed at joint pathophysiology, there is a frustrating dissonance to an approach that separates the disease process from pain. As Nortin Hadler wrote in his 1992 editorial “Knee Pain Is the Malady, Not Osteoarthritis,” “What physician can see an osteophyte on a radiograph without inferring that it reflects the process that is causing the knee to hurt?”28(p598) Indeed, what patient, having been diagnosed with knee OA, can truly be expected to treat the disease process as conceptually separate from the experience of pain and disability? Clearly, the 2 are intertwined. However, the definition of the relevant components of painful knee OA and the interpretation of those components in a heterogeneous population must be the focus of continued research. Pain in knee OA might be considered a “black box.” Many factors have been identified that have relevance to explaining variability in the pain experience (see examples in Fig. 1). Each of these factors may or may not be relevant for any individual patient, and multiple factors may interact to influence pain. Although two patients might possess the same medical diagnosis of knee OA, the contributions to the presence and severity of pain at the individual level remain largely unknown (Fig. 1). Thus, Hadler's 1992 conclusion may still hold true today: “The epidemiology of osteoarthritis and the epidemiology of knee pain have little in common, not nothing in common, but surprisingly little in common.”28(p598) Research over the last 2 decades has provided vast amounts of information illuminating the complexity of pain in knee OA. The task at hand to attempt to harness this complexity to resolve the dissonance inherent to the clinical experience of knee pain for patients diagnosed with knee OA.
Figure 1.
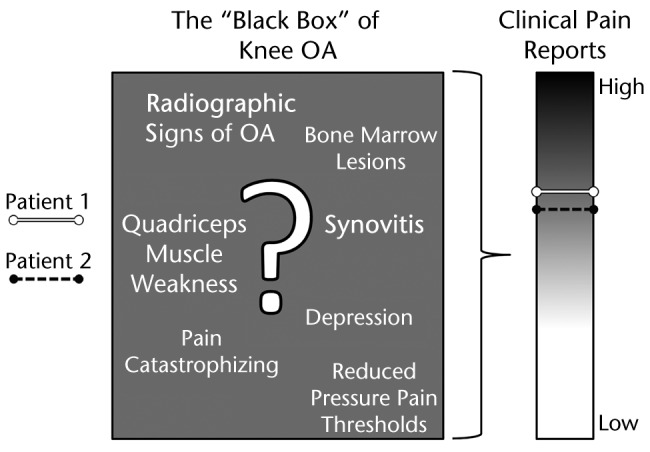
The pain experience in knee osteoarthritis (OA) is a “black box.” Pain appears to be influenced by certain factors—we have provided some examples in this diagram—but the precise contributions to an individual patient's pain experience remain unknown.
Phenotyping Pain in Knee OA
In a 2011 statement to the Food and Drug Administration, the Osteoarthritis Research Society International (OARSI) identified “phenotyping” of OA pain as a research priority with the goal of “better targeting pain therapies to individual patients.”29(p480) This approach aligns with the Institute of Medicine's recent report Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research, which promotes the idea of “tailoring pain care to each person's experience.”30(p2) Both of these directives carry an implicit acknowledgment that previous strategies—based largely on an anatomical, pathophysiological understanding of pain mechanisms—have tended to adopt a one-size-fits-all approach, whereas the clinical pain experience tends to be complex and individualized. However, the process of understanding heterogeneity in the context of knee OA–related pain is only just beginning. Although evidence from large-scale databases suggests that distinct phenotypes exist in knee OA,31 these phenotypes have been described based on a variety of clinical and research measures that are not necessarily specific to pain. The symptoms of knee OA undoubtedly extend beyond pain and can include stiffness, knee instability, and functional deficits, yet pain remains a key feature of the clinical knee OA experience, both as a criterion for diagnosis and as a major driver of health care decisions.14 Of the multiple variables under the knee OA diagnostic umbrella, prioritizing relevance to the pain experience should be an important step in the phenotyping process.
In general, it has been suggested that researching phenotypes for complex musculoskeletal pain conditions should be an intentional process, involving a series of development and validation studies, according to the following phases: (1) selection of appropriate assessment tools, (2) determination and characterization of phenotypes in cross-sectional and longitudinal studies, and (3) validation of proposed phenotypes in both research and clinical settings.32 In this perspective article, we present a model for pain in knee OA that may be used as a broad framework for guiding each of the phases of phenotyping research. This model conceptualizes the “black box” of clinical pain in knee OA as a confluence of variables related to: (1) knee pathology, (2) psychological distress, and (3) the neurological processing of pain (Fig. 2). Our model shares similarities with the model of Diatchenko et al,33 designed to explain the etiology of idiopathic pain disorders, in which the interaction of biological, psychological, and genetic factors was proposed to increase the risk of pain.
Figure 2.
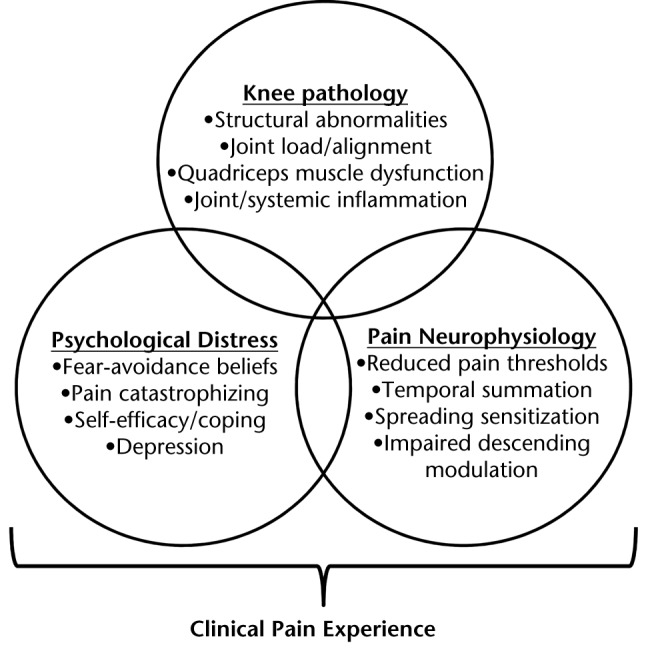
A conceptual model for pain in knee osteoarthritis, emphasizing important contributions from each of the following domains: (1) knee osteoarthritis pathology, (2) psychological distress, and (3) neurophysiological changes in the processing of pain. Variables from these domains (examples provided) are proposed to interact to result in an individual clinical pain experience.
Our intention in proposing this model, with these particular domains, is 2-pronged. First, we believe it is important to prioritize future research and clinical strategies specifically around pain. This focus represents a paradigm shift from previous efforts to understand OA pathophyisiology in isolation. Second, we have selected domains that—in our opinion—offer high potential to provide a more complete description of the pain experience. Although the pathological components of OA have been well studied, psychological and neurophysiological factors have garnered growing interest for their potential influence on the experience of knee pain. Some research and review articles in the last few years have specifically discussed the importance of psychological and neurophysiological factors in painful knee OA.34–36
In the following paragraphs, we will review variables that we believe are candidates for inclusion in this model. We will focus primarily on factors that are likely to be modifiable through therapeutic intervention, although the interested reader might identify other contributors, such as genetic or demographic factors (eg, age, sex) that have the potential to influence knee pain. We do not intend to neglect the role of such factors; we simply believe that a nascent conceptual model such as the one we propose retains some measure of interpretability (and thus usefulness in research and clinical practice) by focusing on avenues for potential intervention. Furthermore, we believe that characterizing pain “phenotypes” in knee OA will present new research opportunities to examine genetic or environmental factors more specifically, as the current phenotypic definition of OA is somewhat vague and problematic.17 Future research will be needed to identify the assessment tools most relevant to knee pain, and there undoubtedly will be opportunities to refine or improve upon our descriptions. As mentioned, the overall goal of this perspective article is to broaden the scope of what is considered important in clinical knee OA to include factors across multiple domains—peripheral, psychological, and neurological—thus allowing for a more complete appreciation of what constitutes a clinically meaningful pain experience.
Harnessing Complexity
Part 1: Knee Pathology
The archetype of knee OA—the older adult with knee pain and bony deformity—remains a common clinical presentation. The conflation of this presentation with the pathophysiology of cartilage degradation may be overly simplistic, but it is an understandable consequence of the utilization of radiography in early attempts to characterize the disease. However, many important nociception-generating processes are not visible on radiographic examination, and knee structures other than cartilage and periarticular bone also have been linked to the pain experience. For example, magnetic resonance imaging (MRI) has identified relationships between pain and synovial thickening, effusion, bone marrow lesions, and meniscal tears.37 As with radiography, these structural abnormalities fail to fully account for pain severity in knee OA,14 and each of the aforementioned MRI findings is frequently found in people with neither pain nor radiographic evidence of degeneration.38 The question that remains is whether structural abnormalities, either in isolation or in combination with other factors, act to influence pain for an individual patient or subset of people diagnosed with knee OA.
One possibility is that imaging abnormalities might serve as a convenient surrogate marker of the potential for nociception at the knee joint, but nociception itself might remain absent or subclinical until influenced by certain biochemical or neurophysiological processes. For example, proinflammatory cytokines are known to sensitize nociceptors, and although OA has classically been considered a noninflammatory condition, evidence exists for both systemic and joint-specific inflammation in people diagnosed with knee OA.39,40 Joint effusion also is often observed clinically and on MRI examination, and a variety of chemical inflammatory mediators have been implicated in this process. In particular, levels of interleukin (IL)-6 and IL-8 appear to be significantly higher in patients with OA compared with controls.39 Both serum and synovial fluid levels of IL-6 and IL-8 demonstrate a relationship with joint fluid and swelling in knee OA.39 Other inflammatory factors, including IL-1 beta and tumor necrosis factor (TNF)-alpha, have been implicated in the pathophysiology of cartilage destruction and nociceptor sensitization.41–43 Interestingly, in a study of the progression of knee pain over 5 years, baseline levels of TNF-alpha, C-reactive protein, and IL-6 were predictive of worse pain at follow-up, even after controlling for radiographic or MRI findings.40 One intriguing possibility, requiring further investigation, is that signs of systemic inflammation represent a common denominator to a number of chronic conditions, including heart disease, diabetes, obesity, and OA.7,44–46 Thus, the presence of multiple inflammatory comorbidities might be helpful in identifying a clinical phenotype of knee OA. However, the mechanisms by which inflammatory processes mediate pain, inflammation, or other signs of disease progression in OA are not well understood—for example, IL-6 has been shown to have anti-inflammatory properties in addition to its documented role in promoting or facilitating inflammation.47
Many of these attempts to characterize the disease of knee OA offer a snapshot of pathophysiology but do not account for activity or load-dependent changes in pain that often accompany the clinical presentation. Weakness of the quadriceps muscle—considered to be a hallmark of knee OA—appears to be strongly related to the presence of pain,48 and the lack of an ability to attenuate forces surrounding the knee joint could play an important role in the experience of knee pain.49–53 Significant correlations also have been observed between pain severity and kinematic and kinetic measures of lower extremity loading.54,55 Malalignment of the tibiofemoral joint, in combination with high body mass index (BMI) may be particularly associated with disease progression in knee OA.56,57
Part 2: Psychological Distress
There has been considerable research regarding psychological influences on the experience of pain and the development of chronic pain (for a recent review, see Linton and Shaw58). Psychological processes such as attention to pain, contextual beliefs about the nature and importance of a particular noxious stimulus, and certain behavioral responses to pain are all thought to be functionally important to any pain experience.59,60 Various models have been proposed to conceptualize how these processes might contribute to chronic pain, and at least a few of these models appear to have particular relevance to pain in knee OA. For example, self-efficacy describes a person's ability to cope and self-manage around a pain experience. Self-efficacy is associated with coping behaviors and beliefs regarding the controllability of pain. Pain coping skills can be taught to patients with arthritis, resulting in increased self-efficacy,61 and there is evidence that high levels of self-efficacy and successful self-management strategies (eg, via exercise or activity modification) are associated with improved pain levels and better functional outcomes.62
The fear-avoidance model of chronic pain—studied at length in patients with low back pain—suggests that the emotional fear of pain, pain-related anxiety, and hyper-attentiveness to pain (among other things) may contribute to maladaptive movement avoidance and inactivity, which subsequently may exacerbate a cycle of worsening pain. The application of this model to knee OA has an intuitive appeal; it is easy to imagine how the diagnosis of “knee OA,” perhaps connoting a progressive, degenerative condition in the minds of patients, could elicit fear and anxiety. Measures of psychological distress—including depression, pain catastrophizing, and pain-related fear, all of which are implicated in the fear-avoidance model—also appear to be influential in the pain experience for patients diagnosed with knee OA. These measures often appear more strongly related to pain than radiographic severity of OA.50,63 Emerging evidence suggests that these measures may be particularly important for establishing prognosis or predicting treatment response. For example, preoperative measures of depression and pain catastrophizing have been shown to predict continued pain after knee replacement surgery.64–66
Part 3: Pain Neurophysiology
Pain is a perception, a construct of the nervous system, not equivalent to (although likely influenced by) sensory nociceptive input from the periphery. Nociception is known to undergo modulation at multiple locations in afferent pathways, and there are opportunities at each of these locations for clinically relevant changes in the neurophysiological processing of pain. In knee OA, the areas of the central nervous system that appear to play a role in the perception and modulation of pain include (but probably are not limited to) the spinal cord, brain stem, thalamus, somatosensory and motor cortices, prefrontal cortex, cingulate cortex, and limbic system.67–69 With chronic knee pain, the overall gain of this system is thought to increase, likely via plastic changes in synaptic connections throughout the relevant networks, resulting for some patients in a central nervous system sensitized to pain. As in other chronic pain conditions, central sensitization in knee OA is hypothesized to constitute a clinical condition unto itself,35 and research is now under way to characterize and address central sensitization in knee OA as an entity separate from pathology at the knee joint.70
Various experimental measures have been used to detect central sensitization in knee OA. These measures are designed to allow researchers and clinicians to rule in a diagnosis of central sensitization by identifying signs of systemic hyperalgesia, by looking for reduced pain thresholds or increased temporal summation of pain, or by characterizing enlarged areas of referral pain or detecting impairments in descending antinociceptive processes. Recently, Arendt-Neilsen et al71 demonstrated reductions in pressure pain threshold in patients with knee OA compared with a control group of individuals who were healthy, both at the knee joint and at anatomically distant sites, suggesting the presence of central sensitization to noxious mechanical stimuli in patients with knee OA. Patients with knee OA also reported increased areas of referred pain in response to hypertonic saline injections.71 Some studies have demonstrated a tendency for patients with knee OA to report increasing levels of pain with the repetitive application of the same noxious stimulus (so-called “temporal summation of pain,” thought to be indicative of nociceptive amplification at the level of the spinal cord).71,72 Descending modulation of nociception, via brain structures such as the rostral ventral medulla and periaqueductal gray, also appears to be disrupted in people with OA.71–74 Some patients with knee OA have been shown to demonstrate symptoms such as fatigue, sleep disturbance, and cognitive difficulties, which also are thought to be indicative of a centrally driven pain experience.75
Future Directions
We propose that variables from peripheral, psychological, and neurophysiological domains should be considered equally important to understanding the individual clinical pain experience for patients with knee OA. In particular, we believe that measures from each of these domains might be used to construct “pain phenotypes” in order to guide future clinical strategies. In our view, such phenotypes would be valuable to at least 3 aspects of clinical practice: (1) diagnosis—through this phenotyping approach, diagnostic categories may be made more relevant to the experience of pain; (2) prognosis—pain phenotypes could be examined longitudinally to characterize potentially different natural histories; and (3) treatment—phenotypes may demonstrate differential responses to currently available treatment strategies, and novel treatment strategies may be developed to target the confluence of factors unique to a particular phenotype.
Diagnosis
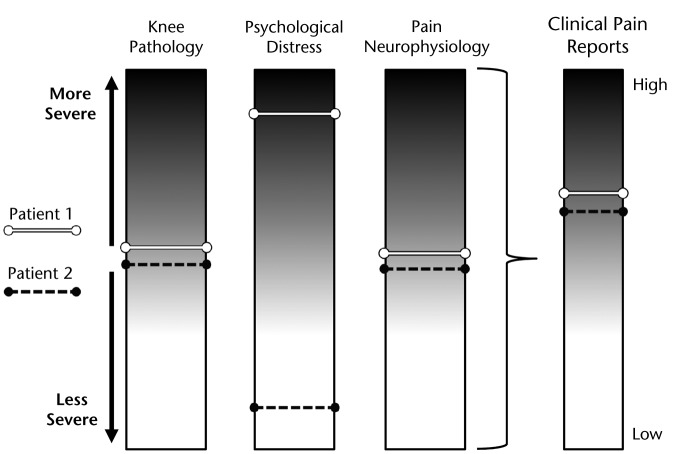
Given the number of measures and, therefore, the complexity of potential interactions among peripheral, psychological, and neurophysiological domains, characterization of pain phenotypes could lead to new diagnostic criteria for knee OA with considerably more relevance to pain. As mentioned, the diagnosis “knee OA” currently relies heavily on radiographic evidence of osteoarthritic changes in the joint, with the rationale that joint degeneration is the primary contributor to the experience of knee pain. This diagnosis has resulted in the identification of pathophysiological elements (eg, cartilage loss, changes to synovium and surrounding tissues) that appear to have an inconsistent relationship to pain. The knee OA diagnostic umbrella—having grown to assimilate multiple elements of OA pathophysiology—now encompasses a heterogeneous population with a wide range of impairments that do not necessarily imply a particular course of physical therapy treatment. However, the diagnosis of pain phenotypes in knee OA, even in the absence of evidence on prognosis or treatment response, may help to guide clinical decision making. For example, a patient with a presentation of moderate levels of structural joint involvement and high levels of psychological distress (patient 1, Fig. 3) appears to warrant a different management strategy than a patient with moderate levels of peripheral joint involvement and low levels of psychological distress (patient 2, Fig. 3). Physical therapists are already accustomed to modifying treatment approaches to address scenarios such as this, perhaps most notably for populations with well-documented psychosocial comorbidity (eg, patients with low back or neck pain). The identification of pain phenotypes may help to formalize similar clinical pathways for physical therapy treatment of knee pain in patients diagnosed with knee OA.
Figure 3.
Patients may present with similar pain reports but with different pain phenotypes. Patient 1 demonstrates moderate levels of joint involvement with high levels of psychological distress. Patient 2 demonstrates moderate levels of joint involvement with low levels of psychological distress. These 2 patients would likely warrant different treatment approaches.
Prognosis
Currently, pain prognosis in knee OA appears to be highly variable. Although many people experience worsening degeneration and worsening pain over a period of time, approximately 30% of people report improvements in knee pain even after several years have elapsed.76 Previous prognostic studies have tended to adopt a biomedical, pathophysiological perspective on knee OA, focusing more on predictors of radiographic degeneration or “disease progression” than on changes in pain.77,78 Still, there is evidence that psychosocial factors (measured at baseline) affect changes in pain status. Preoperative depression was predictive of worse pain following total knee arthroplasty (TKA),64,65 and social deprivation was predictive of worsening knee pain over 7 years in one cohort study.76 Interestingly, this study also identified hypertension, comorbid cardiovascular disease, and BMI as predictors of progressing pain.76
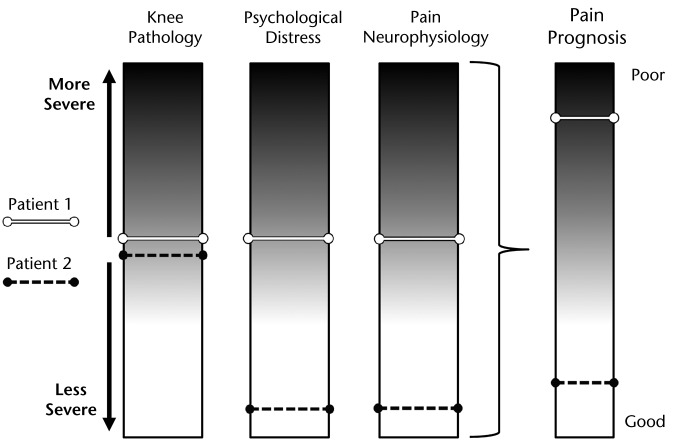
Identification of phenotypes could further our understanding of pain prognosis in knee OA. The interaction of variables across domains, which should be considered in the phenotyping process, appears likely to influence prognosis. For example, it may be that moderate levels of joint involvement and moderate levels of psychological distress, in combination with altered signs of pain neurophysiology, (patient 1, Fig. 4) predispose patients to worse pain over time (ie, the variables have an additive effect on pain presentation or pain prognosis) compared with patients possessing only apparent joint involvement (patient 2, Fig. 4). Currently, the number of potential interactions prohibits these multivariable predictive analyses in all but the largest available datasets (which, in turn, may not contain all of the variables of interest). Therefore, characterization of pain phenotypes could offer a helpful intermediary. Each pain phenotype could act as a surrogate marker for multiple factors, thus simplifying the vast array of potential interactions that would have to be examined longitudinally into a single categorical variable. Future research then could focus on validating knee OA pain phenotypes over time (ie, assessing phenotype stability) and exploring the influence of phenotype on prognosis to determine whether phenotypic variations help explain the variability that has been observed in knee pain prognosis.
Figure 4.
Pain phenotype could influence patients' prognosis. Patient 1 demonstrates moderate levels of joint involvement, in combination with moderate psychological distress and moderate signs of altered pain neurophysiology. Patient 2 demonstrates moderate levels of joint involvement with low levels of psychological distress and minimal changes to pain neurophysiology. In this depiction, involvement of multiple variables across domains has a cumulative effect, leading to poor prognosis.
Treatment
Current approaches to treating the pain associated with knee OA have demonstrated variable success rates. Even the most effective intervention (ie, TKA) may not relieve pain for up to 34% of patients.2 Certain psychosocial factors (namely, depression and pain catastrophizing)64–66,79,80 have been identified as preoperative risk factors for continued pain following TKA, prompting the idea that alternative treatments or rehabilitation strategies may be warranted for individual patients with these psychosocial comorbidities. Recently, Riddle et al81 demonstrated that coping strategies training prior to TKA, for individuals with high levels of pain catastrophizing, resulted in improved pain outcomes following surgery compared with a previous cohort of similar patients. This encouraging, albeit preliminary, finding suggests that treatment approaches might be combined to optimize reductions in pain for patients diagnosed with knee OA. The study by Riddle et al also demonstrated how interventions might be tested in a subpopulation of patients to reduce heterogeneity and maximize apparent treatment effects. Further investigation along these lines is a topic of ongoing research.82 Objective characterization of pain phenotypes could serve to augment these efforts, as additional variables (other than catastrophizing and joint damage) also might be targeted with combined treatment approaches.
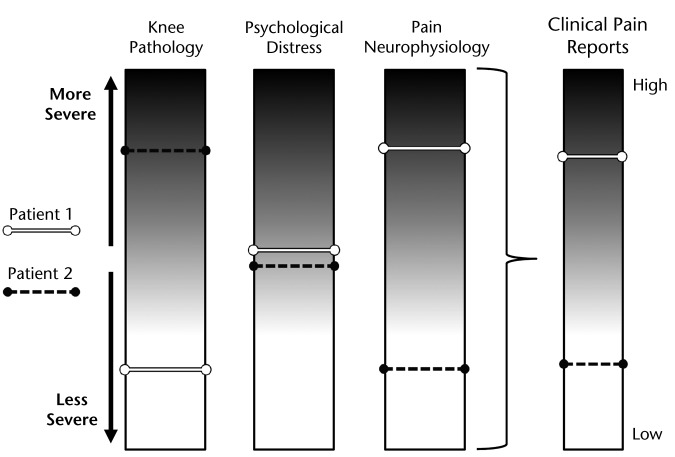
Phenotyping efforts that examine the interaction of variables across domains may help to prioritize our understanding of the impairments most relevant to pain, thereby prompting investigations into novel treatment strategies to address these impairments (or combinations of impairments). For example, a recent study investigated the combined influence of radiographic OA and signs of central sensitization on pain reports for patients with knee OA.83 Mild radiographic OA in conjunction with signs of central sensitization was shown to result in reports of higher levels of pain (exemplified by patient 1, Fig. 5), whereas patients with severe radiographic OA, lacking signs of central sensitization, tended to report lower levels of pain (exemplified by patient 2, Fig. 5). These findings suggest that the changes in the neurophysiological processing of pain have the potential to augment or inhibit the pain experience resulting from radiographic indicators of joint damage. Such knowledge may lead physical therapists to select treatment strategies that harness patients' innate ability to modulate nociception. A patient who possesses the capacity to modulate nociceptive information might benefit from interventions that involve a short-term increase in pain—through manual therapy techniques or aggressive strengthening interventions—to achieve long-term benefits. Conversely, patients who lack the ability to endogenously modulate nociception might warrant strategies that seek to diminish sensitivity of the nervous system, through modalities such as transcutaneous electrical nerve stimulation or other emerging techniques.84,85
Figure 5.
The clinical report of pain may be modified by variables in each domain. Patient 1 demonstrates how the presence of central sensitization might serve to augment the pain resulting from apparently low levels of joint involvement. Patient 2 demonstrates the capacity for the nervous system to modulate pain, diminishing the pain experience in cases of severe joint involvement.
Again, future research will be needed to operationalize our understanding of pain phenotypes based on multiple factors across domains. By empirically characterizing phenotypes from a diagnostic standpoint (and perhaps most productively via data-driven approaches, including specific subgrouping analytical techniques such as those that have been used in other chronic pain populations86–90), physical therapists ultimately may be better equipped to address individual rehabilitation needs across the heterogeneous diagnosis of knee OA.
Conclusion
The painful, deformed knee joint often seen in older adults remains a common clinical presentation. Knee pain continues to affect 1 in 4 older adults, it is commonly reported as a major health issue, and it is a major driver of knee-related health care, including the desire to undergo joint replacement surgery.91 Efforts to define this condition have—over the years—resulted in an impressive list of variables related to structural, biochemical, and mechanical abnormalities of the knee joint that have come to define the pathophysiology of knee OA. Measures related to psychological distress and the neurophysiological processing of pain have recently garnered growing relevance to our understanding of knee pain, but our ability to incorporate this knowledge into the process of diagnosis, prognosis, and treatment remains a work in progress. In many ways, the pain experience in knee OA remains a “black box”; there are factors known to be important, but the essential components of any individual pain experience are unknown.
A new conceptual framework is needed—one that acknowledges the heterogeneity in clinical knee OA and seeks to characterize interactions between variables across multiple domains related to the pain experience. We have proposed a model where knee pain in people diagnosed with OA is composed of variables from the following domains: (1) knee pathology, (2) psychological distress, and (3) pain neurophysiology. We believe that specific combinations of variables across domains will help define different phenotypes of pain. Shifting the emphasis from the mechanisms of joint pathophysiology toward a greater understanding of how combinations of factors influence pain from knee OA ultimately may provide: (1) clinical diagnostic techniques that consider factors beyond joint degeneration, (2) a better sense of patient prognosis and how these factors interact to determine outcomes, and (3) ideas for novel interventions that are matched to the pain phenotype and, therefore, are specific to the individual's pain experience.
Footnotes
All authors provided concept/idea/project design. Dr Kittelson and Dr Stevens-Lapsley provided writing. Dr George, Dr Maluf, and Dr Stevens-Lapsley provided consultation (including review of manuscript before submission).
This work was supported by National Institutes of Health (NIH) grants through the National Institute on Aging and the National Institute of Arthritis and Musculoskeletal Disorders (NIH K23 AG029978, NIH T32 AG000279, NIH R21AG044710, and NIH R01 AR056704). Dr Kittelson received support from the Foundation for Physical Therapy with a Promotion of Doctoral Studies I (PODS I) Award.
References
- 1. Bjordal JM, Ljunggren AE, Klovning A, Slordal L. Non-steroidal anti-inflammatory drugs, including cyclo-oxygenase-2 inhibitors, in osteoarthritic knee pain: meta-analysis of randomised placebo controlled trials. BMJ. 2004;329:1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beswick AD, Wylde V, Gooberman-Hill R, et al. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open. 2012;2:e000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jorgensen A, Stengaard-Pedersen K, Simonsen O, et al. Intra-articular hyaluronan is without clinical effect in knee osteoarthritis: a multicentre, randomised, placebo-controlled, double-blind study of 337 patients followed for 1 year. Ann Rheum Dis. 2010;69:1097–1102 [DOI] [PubMed] [Google Scholar]
- 4. Schappert SM, Burt CW. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 2001–02. Vital Health Stat 13. 2006;(159):1–66 [PubMed] [Google Scholar]
- 5. Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991–94. J Rheumatol. 2006;33:2271–2279 [PubMed] [Google Scholar]
- 6. McAlindon TE, Cooper C, Kirwan JR, Dieppe PA. Knee pain and disability in the community. Br J Rheumatol. 1992;31:189–192 [DOI] [PubMed] [Google Scholar]
- 7. Nuesch E, Dieppe P, Reichenbach S, et al. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ. 2011;342:d1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weiss JM, Noble PC, Conditt MA, et al. What functional activities are important to patients with knee replacements? Clin Orthop Relat Res. 2002;404:172–188 [DOI] [PubMed] [Google Scholar]
- 9. Schellevis FG, van de Lisdonk E, van der Velden J, et al. Validity of diagnoses of chronic diseases in general practice: the application of diagnostic criteria. J Clin Epidemiol. 1993;46:461–468 [DOI] [PubMed] [Google Scholar]
- 10. Spector TD, Dacre JE, Harris PA, Huskisson EC. Radiological progression of osteoarthritis: an 11-year follow-up study of the knee. Ann Rheum Dis. 1992;51:1107–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chapple CM, Nicholson H, Baxter GD, Abbott JH. Patient characteristics that predict progression of knee osteoarthritis: a systematic review of prognostic studies. Arthritis Care Res (Hoboken). 2011;63:1115–1125 [DOI] [PubMed] [Google Scholar]
- 12. Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights, part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–646 [DOI] [PubMed] [Google Scholar]
- 13. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang W, Doherty M, Peat G, et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis. 2010;69:483–489 [DOI] [PubMed] [Google Scholar]
- 15. Neogi T, Felson DT, Niu J, et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ. 2009;339:b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005;365:965–973 [DOI] [PubMed] [Google Scholar]
- 18. Shane Anderson A, Loeser RF. Why is osteoarthritis an age-related disease? Best Pract Res Clin Rheumatol. 2010;24:15–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brandt KD, Fife RS, Braunstein EM, Katz B. Radiographic grading of the severity of knee osteoarthritis: relation of the Kellgren and Lawrence grade to a grade based on joint space narrowing, and correlation with arthroscopic evidence of articular cartilage degeneration. Arthritis Rheum. 1991;34:1381–1386 [DOI] [PubMed] [Google Scholar]
- 20. Spector TD, Cooper C. Radiographic assessment of osteoarthritis in population studies: whither Kellgren and Lawrence? Osteoarthritis Cartilage. 1993;1:203–206 [DOI] [PubMed] [Google Scholar]
- 21. Chan WP, Lang P, Stevens MP, et al. Osteoarthritis of the knee: comparison of radiography, CT, and MR imaging to assess extent and severity. AJR Am J Roentgenol. 1991;157:799–806 [DOI] [PubMed] [Google Scholar]
- 22. Lachance L, Sowers M, Jamadar D, et al. The experience of pain and emergent osteoarthritis of the knee. Osteoarthritis Cartilage. 2001;9:527–532 [DOI] [PubMed] [Google Scholar]
- 23. Kinds MB, Welsing PM, Vignon EP, et al. A systematic review of the association between radiographic and clinical osteoarthritis of hip and knee. Osteoarthritis Cartilage. 2011;19:768–778 [DOI] [PubMed] [Google Scholar]
- 24. Lanyon P, O'Reilly S, Jones A, Doherty M. Radiographic assessment of symptomatic knee osteoarthritis in the community: definitions and normal joint space. Ann Rheum Dis. 1998;57:595–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Felson DT, McAlindon TE, Anderson JJ, et al. Defining radiographic osteoarthritis for the whole knee. Osteoarthritis Cartilage. 1997;5:241–250 [DOI] [PubMed] [Google Scholar]
- 26. Kuettner KE, Goldberg VM. Introduction. In: Kuettner KE, Goldberg VM, eds. Osteoarthritic Disorders. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1995:xxi–xxv [Google Scholar]
- 27. Brandt KD, Dieppe P, Radin E. Etiopathogenesis of osteoarthritis. Med Clin North Am. 2009;93:1–24, xv [DOI] [PubMed] [Google Scholar]
- 28. Hadler NM. Knee pain is the malady: not osteoarthritis. Ann Intern Med. 1992;116:598–599 [DOI] [PubMed] [Google Scholar]
- 29. Lane NE, Brandt K, Hawker G, et al. OARSI-FDA initiative: defining the disease state of osteoarthritis. Osteoarthritis Cartilage. 2011;19:478–482 [DOI] [PubMed] [Google Scholar]
- 30. Committee on Advancing Pain Research, Care, and Education, Institute of Medicine Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011 [PubMed] [Google Scholar]
- 31. Knoop J, van der Leeden M, Thorstensson CA, et al. Identification of phenotypes with different clinical outcomes in knee osteoarthritis: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken). 2011;63:1535–1542 [DOI] [PubMed] [Google Scholar]
- 32. Kent P, Keating JL, Leboeuf-Yde C. Research methods for subgrouping low back pain. BMC Med Res Methodol. 2010;10:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Diatchenko L, Nackley AG, Slade GD, et al. Idiopathic pain disorders: pathways of vulnerability. Pain. 2006;123:226–230 [DOI] [PubMed] [Google Scholar]
- 34. Sluka KA, Berkley KJ, O'Connor MI, et al. Neural and psychosocial contributions to sex differences in knee osteoarthritic pain. Biol Sex Differ. 2012;3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lluch Girbés E, Nijs J, Torres-Cueco R, López Cubas C. Pain treatment for patients with osteoarthritis and central sensitization. Phys Ther. 2013;93:842–851 [DOI] [PubMed] [Google Scholar]
- 36. Somers TJ, Keefe FJ, Godiwala N, Hoyler GH. Psychosocial factors and the pain experience of osteoarthritis patients: new findings and new directions. Curr Opin Rheumatol. 2009;21:501–506 [DOI] [PubMed] [Google Scholar]
- 37. Torres L, Dunlop DD, Peterfy C, et al. The relationship between specific tissue lesions and pain severity in persons with knee osteoarthritis. Osteoarthritis Cartilage. 2006;14:1033–1040 [DOI] [PubMed] [Google Scholar]
- 38. Guermazi A, Niu J, Hayashi D, et al. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study). BMJ. 2012;345:e5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kaneko S, Satoh T, Chiba J, et al. Interleukin-6 and interleukin-8 levels in serum and synovial fluid of patients with osteoarthritis. Cytokines Cell Mol Ther. 2000;6:71–79 [DOI] [PubMed] [Google Scholar]
- 40. Stannus OP, Jones G, Blizzard L, et al. Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: a prospective cohort study. Ann Rheum Dis. 2013;72:535–540 [DOI] [PubMed] [Google Scholar]
- 41. Caron JP, Fernandes JC, Martel-Pelletier J, et al. Chondroprotective effect of intraarticular injections of interleukin-1 receptor antagonist in experimental osteoarthritis: suppression of collagenase-1 expression. Arthritis Rheum. 1996;39:1535–1544 [DOI] [PubMed] [Google Scholar]
- 42. van de Loo FA, Joosten LA, van Lent PL, et al. Role of interleukin-1, tumor necrosis factor alpha, and interleukin-6 in cartilage proteoglycan metabolism and destruction: effect of in situ blocking in murine antigen- and zymosan-induced arthritis. Arthritis Rheum. 1995;38:164–172 [DOI] [PubMed] [Google Scholar]
- 43. Schaible HG, von Banchet GS, Boettger MK, et al. The role of proinflammatory cytokines in the generation and maintenance of joint pain. Ann N Y Acad Sci. 2010;1193:60–69 [DOI] [PubMed] [Google Scholar]
- 44. Couzin-Frankel J. Inflammation bares a dark side. Science. 2010;330:1621. [DOI] [PubMed] [Google Scholar]
- 45. Presle N, Pottie P, Dumond H, et al. Differential distribution of adipokines between serum and synovial fluid in patients with osteoarthritis: contribution of joint tissues to their articular production. Osteoarthritis Cartilage. 2006;14:690–695 [DOI] [PubMed] [Google Scholar]
- 46. Rojas-Rodriguez J, Escobar-Linares LE, Garcia-Carrasco M, et al. The relationship between the metabolic syndrome and energy-utilization deficit in the pathogenesis of obesity-induced osteoarthritis. Med Hypotheses. 2007;69:860–868 [DOI] [PubMed] [Google Scholar]
- 47. Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–888 [DOI] [PubMed] [Google Scholar]
- 48. O'Reilly SC, Jones A, Muir KR, Doherty M. Quadriceps weakness in knee osteoarthritis: the effect on pain and disability. Ann Rheum Dis. 1998;57:588–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hall MC, Mockett SP, Doherty M. Relative impact of radiographic osteoarthritis and pain on quadriceps strength, proprioception, static postural sway and lower limb function. Ann Rheum Dis. 2006;65:865–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. O'Reilly SC, Jones A, Muir KR, Doherty M. Quadriceps weakness in knee osteoarthritis: the effect on pain and disability. Ann Rheum Dis. 1998;57:588–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Callaghan MJ, Oldham JA. Quadriceps atrophy: to what extent does it exist in patellofemoral pain syndrome? Br J Sports Med. 2004;38:295–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. van der Worp H, van Ark M, Roerink S, et al. Risk factors for patellar tendinopathy: a systematic review of the literature. Br J Sports Med. 2011;45:446–452 [DOI] [PubMed] [Google Scholar]
- 53. Kaya D, Citaker S, Kerimoglu U, et al. Women with patellofemoral pain syndrome have quadriceps femoris volume and strength deficiency. Knee Surg Sports Traumatol Arthrosc. 2011;19:242–247 [DOI] [PubMed] [Google Scholar]
- 54. Thorp LE, Sumner DR, Wimmer MA, Block JA. Relationship between pain and medial knee joint loading in mild radiographic knee osteoarthritis. Arthritis Rheum. 2007;57:1254–1260 [DOI] [PubMed] [Google Scholar]
- 55. Teichtahl AJ, Wluka AE, Morris ME, et al. The relationship between the knee adduction moment and knee pain in middle-aged women without radiographic osteoarthritis. J Rheumatol. 2006;33:1845–1848 [PubMed] [Google Scholar]
- 56. Felson DT, Goggins J, Niu J, et al. The effect of body weight on progression of knee osteoarthritis is dependent on alignment. Arthritis Rheum. 2004;50:3904–3909 [DOI] [PubMed] [Google Scholar]
- 57. Sharma L, Song J, Felson DT, et al. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286:188–195 [DOI] [PubMed] [Google Scholar]
- 58. Linton SJ, Shaw WS. Impact of psychological factors in the experience of pain. Phys Ther. 2011;91:700–711 [DOI] [PubMed] [Google Scholar]
- 59. Turk DC, Melzack R. Handbook of Pain Assessment. 3rd ed New York, NY: Guilford Press; 2011 [Google Scholar]
- 60. Eccleston C, Crombez G. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol Bull. 1999;125:356–366 [DOI] [PubMed] [Google Scholar]
- 61. Lorig KR, Mazonson PD, Holman HR. Evidence suggesting that health education for self-management in patients with chronic arthritis has sustained health benefits while reducing health care costs. Arthritis Rheum. 1993;36:439–446 [DOI] [PubMed] [Google Scholar]
- 62. Lorig KR, Ritter PL, Laurent DD, Plant K. The Internet-based arthritis self-management program: a one-year randomized trial for patients with arthritis or fibromyalgia. Arthritis Rheum. 2008;59:1009–1017 [DOI] [PubMed] [Google Scholar]
- 63. Heuts PH, Vlaeyen JW, Roelofs J, et al. Pain-related fear and daily functioning in patients with osteoarthritis. Pain. 2004;110:228–235 [DOI] [PubMed] [Google Scholar]
- 64. Brander VA, Stulberg SD, Adams AD, et al. Predicting total knee replacement pain: a prospective, observational study. Clin Orthop Relat Res. 2003;416:27–36 [DOI] [PubMed] [Google Scholar]
- 65. Brander VA, Gondek S, Martin E, Stulberg SD. Pain and depression influence outcome 5 years after knee replacement surgery. Clin Orthop Relat Res. 2007;464:21–26 [DOI] [PubMed] [Google Scholar]
- 66. Riddle DL, Wade JB, Jiranek WA, Kong X. Preoperative pain catastrophizing predicts pain outcome after knee arthroplasty. Clin Orthop Relat Res. 2010;468:798–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kulkarni B, Bentley DE, Elliott R, et al. Arthritic pain is processed in brain areas concerned with emotions and fear. Arthritis Rheum. 2007;56:1345–1354 [DOI] [PubMed] [Google Scholar]
- 68. Parks EL, Geha PY, Baliki MN, et al. Brain activity for chronic knee osteoarthritis: dissociating evoked pain from spontaneous pain. Eur J Pain. 2011;15:843: e1–e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lee YC, Nassikas NJ, Clauw DJ. The role of the central nervous system in the generation and maintenance of chronic pain in rheumatoid arthritis, osteoarthritis and fibromyalgia. Arthritis Res Ther. 2011;13:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. O'Neill S, Manniche C, Graven-Nielsen T, Arendt-Nielsen L. Generalized deep-tissue hyperalgesia in patients with chronic low-back pain. Eur J Pain. 2007;11:415–420 [DOI] [PubMed] [Google Scholar]
- 71. Arendt-Nielsen L, Nie H, Laursen MB, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149:573–581 [DOI] [PubMed] [Google Scholar]
- 72. Skou ST, Graven-Nielsen T, Lengsoe L, et al. Relating clinical measures of pain with experimentally assessed pain mechanisms in patients with knee osteoarthritis. Scand J Pain. 2013;4:111–117 [DOI] [PubMed] [Google Scholar]
- 73. Graven-Nielsen T, Wodehouse T, Langford RM, et al. Normalization of widespread hyperesthesia and facilitated spatial summation of deep-tissue pain in knee osteoarthritis patients after knee replacement. Arthritis Rheum. 2012;64:2907–2916 [DOI] [PubMed] [Google Scholar]
- 74. Gwilym SE, Keltner JR, Warnaby CE, et al. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum. 2009;61:1226–1234 [DOI] [PubMed] [Google Scholar]
- 75. Murphy SL, Lyden AK, Phillips K, et al. Association between pain, radiographic severity, and centrally-mediated symptoms in women with knee osteoarthritis. Arthritis Care Res (Hoboken). 2011;63:1543–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Peters TJ, Sanders C, Dieppe PA, Donovan J. Factors associated with change in pain and disability over time: a community-based prospective observational study of hip and knee osteoarthritis. Br J Gen Pract. 2005;55:205–211 [PMC free article] [PubMed] [Google Scholar]
- 77. Dieppe PA, Cushnaghan J, Shepstone L. The Bristol “OA500” study: progression of osteoarthritis (OA) over 3 years and the relationship between clinical and radiographic changes at the knee joint. Osteoarthritis Cartilage. 1997;5:87–97 [DOI] [PubMed] [Google Scholar]
- 78. Felson DT. The course of osteoarthritis and factors that affect it. Rheum Dis Clin North Am. 1993;19:607–615 [PubMed] [Google Scholar]
- 79. Forsythe ME, Dunbar MJ, Hennigar AW, et al. Prospective relation between catastrophizing and residual pain following knee arthroplasty: two-year follow-up. Pain Res Manag. 2008;13:335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sullivan M, Tanzer M, Stanish W, et al. Psychological determinants of problematic outcomes following total knee arthroplasty. Pain. 2009;143:123–129 [DOI] [PubMed] [Google Scholar]
- 81. Riddle DL, Keefe FJ, Nay WT, et al. Pain coping skills training for patients with elevated pain catastrophizing who are scheduled for knee arthroplasty: a quasi-experimental study. Arch Phys Med Rehabil. 2011;92:859–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Riddle DL, Keefe FJ, Ang D, et al. A phase III randomized three-arm trial of physical therapist delivered pain coping skills training for patients with total knee arthroplasty: the KASTPain protocol. BMC Musculoskelet Disord. 2012;13:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Finan PH, Buenaver LF, Bounds SC, et al. Discordance between pain and radiographic severity in knee osteoarthritis findings from quantitative sensory testing of central sensitization. Arthritis Rheum. 2013;65:363–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Vance CG, Rakel BA, Blodgett NP, et al. Effects of transcutaneous electrical nerve stimulation on pain, pain sensitivity, and function in people with knee osteoarthritis: a randomized controlled trial. Phys Ther. 2012;92:898–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dailey DL, Rakel BA, Vance CG, et al. Transcutaneous electrical nerve stimulation reduces pain, fatigue and hyperalgesia while restoring central inhibition in primary fibromyalgia. Pain. 2013;154:2554–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Giesecke T, Williams DA, Harris RE, et al. Subgrouping of fibromyalgia patients on the basis of pressure-pain thresholds and psychological factors. Arthritis Rheum. 2003;48:2916–2922 [DOI] [PubMed] [Google Scholar]
- 87. Calandre EP, Garcia-Carrillo J, Garcia-Leiva JM, et al. Subgrouping patients with fibromyalgia according to the results of the Fibromyalgia Impact Questionnaire: a replication study. Rheumatol Int. 2011;31:1555–1559 [DOI] [PubMed] [Google Scholar]
- 88. Oswald J, Salemi S, Michel BA, Sprott H. Use of the Short-Form-36 Health Survey to detect a subgroup of fibromyalgia patients with psychological dysfunction. Clin Rheumatol. 2008;27:919–921 [DOI] [PubMed] [Google Scholar]
- 89. Borsbo B, Peolsson M, Gerdle B. The complex interplay between pain intensity, depression, anxiety and catastrophising with respect to quality of life and disability. Disabil Rehabil. 2009;31:1605–1613 [DOI] [PubMed] [Google Scholar]
- 90. Beneciuk JM, Robinson ME, George SZ. Low back pain subgroups using fear-avoidance model measures: results of a cluster analysis. Clin J Pain. 2012;28:658–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Clark JP, Hudak PL, Hawker GA, et al. The moving target: a qualitative study of elderly patients' decision-making regarding total joint replacement surgery. J Bone Joint Surg Am. 2004;86:1366–1374 [PubMed] [Google Scholar]