Abstract
Numerous externalizing behaviors, from aggression to risk-taking to drug abuse, stem from impaired cognitive control, including that brought about by the acute effects of alcohol. Although research generally indicates that alcohol impairs cognitive abilities, a close examination of the literature suggests that alcohol’s effects are quite variable and likely depend on a number of contextual factors. The purpose of the current study was to characterize the effects of alcohol on cognitive control in terms of neural and behavioral responses to successful and unsuccessful control attempts. Participants were randomly assigned to consume an alcohol (0.80 g/kg ETOH), placebo, or non-alcoholic control beverage prior to completing a cognitive control (flanker) task while event-related brain potentials (ERPs) were recorded. Alcohol reduced the amplitude of the error-related negativity (ERN) on error trials and increased the post-error compatibility effect in response time. Of particular interest, neural indices of conflict monitoring (N2) and performance adjustment (frontal slow wave) were attenuated by alcohol, but only on trials following errors. These functions had recovered, however, by two trials after an error. These findings suggest that alcohol’s effects on cognitive control are best characterized as impaired (or delayed) recovery following control failures. Implications of these findings for understanding alcohol’s effects on behavioral under-control are discussed.
Keywords: alcohol, cognitive control, ERPs, post-error adjustment, externalizing
Alcohol is commonly understood to impair cognitive functioning (see Curtin & Lang, 2007; Giancola, 2000; Sayette, 1999), which has been posited as a major source of the drug’s deleterious effects on externalizing behaviors, including increased aggression (Giancola, 2004; Giancola, Josephs, De Wall, & Gunn, 2009; Godlaski & Giancola, 2009), greater risk-taking (Cherpitel, 2006; Fromme, Katz, & D’Amico, 1997; George, Rogers, & Duka, 2005), and engagement in injurious behaviors (MacDonald, Zanna, & Fong, 1996, 1995). Despite this theory and research, a close examination of the literature indicates that alcohol’s effects on cognition are not uniform. In particular, whereas cognitive processes believed to unfold in a relatively obligatory or automatic fashion seem largely immune to alcohol’s effects (see Bartholow, Dickter, & Sestir, 2006; Fillmore, Vogel-Sprott, & Gavrilescu, 1999; Grattan & Vogel-Sprott, 2001), alcohol seems to especially impair cognitive control (e.g., Bartholow et al., 2006; Casbon, Curtin, Lang, & Patrick, 2003; Curtin & Fairchild, 2003; Fillmore et al., 1999; Pihl, Paylan, Gentes-Hawn, & Hoaken, 2003), a set of higher-order processes important for pursuing goal-directed action (Alexander & Brown, 2010; Carter & van Veen, 2007; Miller & Cohen, 2001). But cognitive control itself is not a single or a simple construct (see Botvinick, Braver, Barch, Carter, & Cohen, 2001; Braver, 2012; Miyake, Friedman, Emerson, Witzki, & Howerter, 2000), and therefore the notion that alcohol ‘impairs cognitive control’ is likely over-simplified as well. The purpose of the current study was to move toward a more specific model of alcohol’s effects on cognitive control by identifying circumstances under which impairment is more likely to occur.
Cognitive Control and Its Impairment by Alcohol
Numerous theoretical models (see Banich et al., 2009; Crocker et al., 2013; Groman, James, & Jentsch, 2009) and empirical demonstrations (e.g., Fillmore & Rush, 2006; Mezzacappa, Kindlon, & Earls, 1999; Romer et al., 2009; Shehzad, DeYoung, Kang, Grigorenko, & Gray, 2012) have underscored the role of cognitive control and its neural correlates in externalizing psychopathology. Specifically, dysfunction in frontal and prefrontal cortical circuits known to underlie cognitive control abilities, such as inhibition, attention control, and planning, are characteristic of individuals who exhibit externalizing problems such as impulsive aggression (e.g., Giancola, 1995; Giancola & Zeichner, 1994), attention-deficit disorder (e.g., Aron & Poldrack, 2005; Itami & Uno, 2002), drug abuse (e.g., Thompson et al., 2004), and risky sexual activity (Miner, Raymond, Mueller, Lloyd, & Lim, 2009). Thus, understanding factors that contribute to impairment in cognitive control and its neural substrates has important implications for elucidating externalizing problems in both clinical and nonclinical populations.
Intoxicated or sober, cognitive control is imperfect in most people. Thus, the ability to monitor ongoing performance and make adjustments when necessary is a critical, adaptive function of the information processing system (see Holroyd & Coles, 2002). Recent theoretical models have emphasized the importance of conflict monitoring and performance adjustment to ensure adequate goal-directed performance (e.g., Botvinick et al., 2001; Braver, 2012; Jacoby, Jennings, & Hay, 1996; Meyer & Kieras, 1997). In situations involving the presence of conflicting response possibilities (i.e., where some stimulus features elicit a response that conflicts with the goal-directed response), control is needed to maintain attention on task goals, to focus attention on task-relevant stimulus features and ignore task-irrelevant features, and to bias motor responding in favor of the correct response.
Considerable research has demonstrated that conflict monitoring can be impaired by alcohol. Building on a long-standing thesis that alcohol most affects behavior under conditions of response conflict (see Steele & Southwick, 1985), Curtin and Fairchild (2003) tested effects of alcohol on conflict monitoring by having participants complete a Stroop task (Stroop, 1935) either intoxicated or sober. These authors reported that alcohol increased Stroop interference and reduced event-related potential (ERP) responses associated with conflict monitoring (N450) and implementation of control (negative slow wave).
Other research has shown, however, that the mere presence of conflict does not ensure that alcohol will impair performance. For example, Bartholow et al. (2003) found that alcohol reduced response accuracy on high-conflict trials in a cognitive control task (the flanker task; Eriksen & Eriksen, 1974) when most of the trials within a block were compatible (low-conflict), but not when most of the trials were incompatible (high-conflict). Other studies similarly have shown no effects of alcohol on cognitive control performance when most or all of the trials within a task are high-conflict (Gustafson & Kallmen, 1990a, 1990b; Tarter, Jones, Simpson, & Vega, 1971), or when participants are sufficiently motivated to perform accurately (Gustafson & Kallmen, 1990c). Finally, providing extensive training on a task can limit the effect of alcohol on performance. Ridderinkhof et al. (2002) found that when participants were trained to achieve a given level of performance in the flanker task (e.g., 15% errors), the size of the compatibility effect (CE; incompatible trial – compatible trial) in response time (RT) was not modulated by alcohol. The CE reflects the extent to which incompatible flankers interfere with processing of the target stimulus (Eriksen & Eriksen, 1974; Eriksen & Hoffman, 1973), so a larger CE reflects greater conflict from peripheral, non-target information. Considered along with prevailing theory (e.g., Giancola, 2004; Steele & Josephs, 1990), such findings suggest that conflict might be a necessary but not a sufficient condition for alcohol to impair cognitive control.
Performance adjustment is the process whereby control is enhanced following the presence of conflict (e.g., Gratton, Coles, Sirevaag, Eriksen, & Donchin, 1988) or when control has failed and an error has occurred (e.g., Garavan, Ross, Murphy, Roche, & Stein, 2002; Rabbitt, 1966; Ridderinkhof, Ullsperger, Crone, & Nieuwenhuis, 2004; Ullsperger & von Cramen, 2006). In particular, the occurrence of errors should signal that the current implementation of control is insufficient and must be adjusted in order to maintain desirable performance (Yeung, Botvinick, & Cohen, 2004). One behavioral index of performance-based adjustments in cognitive control is the size of the CE following incorrect trials. If errors prompt an increase in control, the CE should be smaller following errors than following correct responses, as enhanced control should attenuate the difference in responses to compatible and incompatible trials. This kind of effect has been reported by numerous researchers (e.g., Burle, Passamai, Vidal, Bonnet, & Hasbroucq, 2002; Rabbitt & Vyas, 1970; Smith & Brewer, 1995). Using a flanker task, Ridderinkhof and colleagues (2002) tested whether alcohol impairs post-error adjustment, reporting that the magnitude of the CE was larger following errors than correct responses among participants who had consumed alcohol, indicating that alcohol impairs the ability to enhance control following errors (see also Bartholow, Henry, Lust, Saults, & Wood, 2012).
Thus, alcohol has been shown to impair both conflict monitoring (under some conditions) and post-error performance adjustment. Might these effects emerge only once control has already failed? Close inspection of the sequential trial RT data reported by both Ridderinkhof et al. (2002) and Bartholow et al. (2012) suggests that, whereas alcohol has a pronounced effect on the post-error CE relative to placebo, alcohol does not affect the magnitude of the CE following correct responses. This pattern suggests that alcohol might primarily impair conflict monitoring once control has failed, leading to disruption of downstream regulative control processes thought to rely on conflict detection to signal the need to adjust. According to theory (e.g., Botvinick, 2007), control failures (i.e., errors) during response conflict tasks often indicate that conflict was not sufficiently registered and overcome prior to the response, and instead is registered by the anterior cingulate cortex (ACC) at the time of the response (also see Yeung et al., 2004). This conflict-related ACC response is believed to signal other areas of prefrontal cortex that an increase in control is needed so as to avoid further mistakes, what some have called regulative control (Botvinick et al., 2001; Kerns et al., 2004). Alcohol has been shown to reduce this error-induced ACC activity (Bartholow et al., 2012; Ridderinkhof et al., 2002), as well as neural responses emanating from lateral prefrontal cortical areas thought to underlie regulative control (e.g., frontal slow wave; Bartholow et al., 2006; Curtin & Fairchild, 2003). However, no research has investigated whether neural manifestations of conflict monitoring and adjustment are reduced by alcohol specifically on post-error trials, or whether such effects might underlie alcohol’s impairment of post-error behavioral performance.
Moreover, the possibility that post-error control recovery plays a role in determining alcohol’s effects on cognitive performance has not been investigated. Although post-error control adjustment is clearly impaired by alcohol (Bartholow et al., 2012; Ridderinkhof et al., 2002), it seems unlikely that control cannot be recovered. If so, we would expect alcohol-induced performance deficits to be much more pronounced than they generally are. Rather, it could be that post-error recovery of control simply takes longer than usual under the influence of alcohol. Evidence supporting this hypothesis could suggest new models of alcohol’s effects on problem behaviors (see Giancola, 2000; Lange, 2002), in which moment-to-moment fluctuations in control play a key role.
ERP Measures of Control Processes
ERPs provide a noninvasive, temporally precise way of measuring the implementation of different components of cognitive control during task performance. The commission of errors in choice RT tasks elicits a pronounced negative deflection, the error-related negativity (ERN), prominent at fronto-central midline scalp locations, which coincides with error commission and which is thought to reflect ACC responses to errors (Coles, Scheffers, & Holroyd, 2001; Holroyd & Coles, 2002) and/or conflict (Botvinick, Cohen, & Carter, 2004; Yeung et al., 2004). A role for the processes reflected in the ERN in cognitive control has been suggested by studies showing that the magnitude of the ERN is associated with behavioral control overall (e.g., Amodio, Harmon-Jones, Devine, Hartley, & Covert, 2004; Bartholow et al., 2012), and specifically on trials following errors (Kerns et al., 2004). Alcohol has been shown to reduce ERN amplitude and attenuate these associations, however (Bartholow et al., 2012; Ridderinkhof et al., 2002). In the current study, the ERN was used to signify the effectiveness of conflict monitoring during control failures.
In addition to the response-locked ERN, two stimulus-locked ERP components—the N2 and frontal slow wave (FSW)—are thought to index processes associated with conflict monitoring and performance adjustment, respectively. The N2 is a transient negativity over frontal and frontal-central scalp sites, peaking between 200–350 ms after stimulus onset (see van Veen & Carter, 2002; Yeung et al., 2004). The N2 is highly sensitive to response conflict (e.g., larger during incompatible than compatible flanker trials; Kopp, Rist, & Mattler, 1996), and, similar to the ERN, source localization indicates that the N2 originates in the ACC (van Veen & Carter, 2002; Yeung et al., 2004). Research examining correct-trial performance has shown no effects of alcohol on N2 amplitude (Bartholow et al., 2006; Easdon, Izenberg, Armilio, Yu, & Alain, 2005; Ridderinkhof et al., 2002; Rohrbaugh et al., 1987). No previous study has tested whether the N2 (and the conflict-monitoring process it reflects) is differentially affected by alcohol as a function of trial-to-trial fluctuations in performance, however. In the current study, consistent with the idea that alcohol’s impairment of control can be characterized in terms of deficits in reinstating control following control failures, it was predicted that the CE in N2 amplitude would be attenuated by alcohol on post-error trials but not on trials following correct responses.
The FSW appears as a relatively low-frequency negative deflection developing later in the stimulus-locked epoch in tasks invoking cognitive control (see West & Alain, 1999, 2000). The FSW differentiates correct responses from errors (West & Travers, 2008) and is larger on incongruent than congruent Stroop trials (Bailey, West, & Anderson, 2010; West & Alain, 1999, 2000; West, Bailey, Tiernan, Boonsuk, & Gilbert, 2012). The FSW is thought to reflect activity in the lateral prefrontal cortex related to implementation of or adjustments in control (West & Bailey, 2012; West et al., 2012). Consistent with this idea, studies have demonstrated that FSW amplitude is correlated with behavioral indices of adjustments in control (Bailey et al., 2010; Bartholow et al., 2006), and varies along with the amount of conflict present within a block of trials (West & Bailey, 2012). Previous studies (Bartholow et al., 2006; Curtin & Fairchild, 2003) also have shown that alcohol attenuates FSW amplitude during cognitive control tasks. To our knowledge, however, no previous study has investigated whether the FSW is sensitive to the accuracy of the previous trial. If alcohol’s impairment of control is evident when control adjustments are most needed, FSW amplitude should be attenuated by alcohol on post-error trials, but be unaffected by alcohol on trials that follow correct responses.
The Current Study
The current study was designed to clarify the nature of alcohol’s effects on cognitive control by investigating the extent to which typical performance fluctuations influence conflict monitoring and performance adjustment under sober and intoxicated conditions. Findings from this study have the potential to inform models of alcohol effects on numerous problem behaviors known to be regulated by cognitive control (e.g., aggression, excessive/additional alcohol intake). The study addressed the following questions: 1) Does alcohol affect conflict monitoring and performance adjustment differently as a function of whether or not control failures occur? 2) If alcohol impairs these processes specifically following control failures, how soon does control recover? 3) Are neural signals of conflict monitoring and performance adjustment associated with behavioral manifestations of control, and if so, do these associations differ under intoxicated and sober conditions? Participants performed the arrow flanker task after consuming alcohol, a placebo, or a non-alcoholic control beverage. Behavioral (i.e., RT, accuracy, and posterror CE in RT) and ERP (i.e., ERN, N2, and FSW) indices of performance and cognitive control were examined on trials following correct and incorrect responses. Based on prior work (Bartholow et al., 2012; Ridderinkhof et al., 2002), we expected ERN amplitude to be decreased (indicating reduced conflict monitoring during control failures) and the post-error CE in RT to be increased (indicating impaired performance adjustment) by alcohol. We further hypothesized that the amplitude of the N2 and FSW elicited by high-conflict (incompatible) flanker arrays would be attenuated in the alcohol relative to the control and placebo groups, but only on post-error trials. Finally, we predicted that these indices of control would rebound in the alcohol group on subsequent trials.
Method
Participants
Ninety-six healthy adults (49 women), all white/non-Hispanic, ages 21–36 years (M = 23 years, SD = 3), were recruited from the Columbia, MO community using mass email announcements and advertisements in local periodicals. Eligibility was determined using a structured telephone interview. Individuals indicating any condition that would contraindicate alcohol administration (pregnancy; abstention; symptoms of alcohol or drug dependence; history of serious mental or physical illness; prescription medication other than oral contraception) or who reported history of head trauma or neurological disorder were excluded from the sample, as were individuals who reported drinking less than an average of 2 or more than an average of 25 drinks per week in the past three months. Eligible participants were scheduled for lab appointments and instructed to abstain from alcohol and other drugs for 24 hours prior and to eat a light meal 4–6 hours prior to their appointment. All lab sessions began between 12:00-4:00 pm. Affidavits completed upon arrival at the lab were used to ensure participants’ compliance with pre-session protocols and maintenance of study eligibility since the interview. Participants were compensated $12/hour for their time.
Beverage Administration
Participants were randomly assigned to consume a no-alcohol control beverage (n = 30 [16 women]), an active placebo beverage (n = 33 [18 women]; 0.04 g/kg alcohol), or an alcohol beverage (n = 33 [15 women]; 0.80 g/kg alcohol; target peak breath alcohol concentration [BrAC] = .08%). Participants in the control condition were told that their beverage contained no alcohol; participants in the placebo and alcohol conditions were told that their beverage contained “a moderate amount of alcohol.” In these conditions, an experimenter ostensibly prepared a beverage containing a 5:1, tonic to vodka ratio. The placebo dose was achieved with diluted (10 proof) vodka (9 parts flattened tonic to 1 part 100-proof vodka, poured from a Smirnoff Blue Label® bottle) and tonic; the alcohol dose was achieved using 100-proof vodka and tonic, calculated based on total body water volume (estimated using age, gender, height, and weight) and the duration of the drinking period (15 min), using published formulas (see Curtin & Fairchild, 2003; Watson, 1989). Participants in the control group consumed a tonic-only beverage. Total beverage was isovolemic across conditions. The beverage was divided into three equal-size drinks and participants were given 5 min to consume each one. After the drinking period, participants sat idle for 5 additional min to ensure initial alcohol absorption into the blood prior to starting the task.
Measures
Cognitive control task
Participants completed a flanker task with arrays of right- and left-facing arrows presented inside a horizontal rectangle. On compatible trials, flanker (peripheral) arrows faced in the same direction as the (central) target (i.e., →→→→→ or ←←←←←); on incompatible trials, flanker arrows faced in the opposite direction of the target (i.e., →→←→→ or ←←→←←). Compatible and incompatible arrays were presented pseudo-randomly, with the constraints that they occurred with equal probability and called for left- and right-hand responses equally often. Participants were instructed to respond to left-facing targets by pressing a button with their left index finger and to right-facing targets by pressing a button with their right index finger. The horizontal rectangle remained on the screen throughout the task, shown in black against a light gray (10% black) background. Targets were presented in dark gray (80% black). To strengthen flanker interference, the immediately surrounding arrows were slightly darker (90% black) and 10% larger than the targets, and the outermost flankers were even darker (100% black) and larger (20%) than the targets (see Ridderinkhof et al., 2002). Arrow arrays were presented for 100 ms.
Prior to completing 10 blocks of 80 experimental trials, participants completed 7 blocks of 28 practice trials, during which performance was monitored by the computer program to ensure that each participant attained a speed/accuracy balance that produced approximately 10% errors. Participants making fewer errors were instructed to respond more quickly. No feedback was given during the experimental trials. Given that ERN amplitude decreases as the number of errors increases (see Gehring et al., 1993; but see Olvet & Hajcak, 2009), this comparability in performance ensured that any decrease in ERN amplitude in the alcohol group was not due simply to a larger number of errors in that group relative to the other groups. In addition, and similar to previous studies in which response confidence judgments have been recorded (Bartholow et al., 2012; Scheffers & Coles, 2000), following the response on each trial a 3-point scale appeared on the screen, with anchor points labeled “Sure Correct”, “Don’t Know”, and “Sure Incorrect.” Participants were instructed to indicate their confidence in the correctness of the response they just made by pressing one of three buttons on their button box within 3 seconds. The next trial began following an inter-trial interval that varied randomly between 1100 and 1500ms.
Subjective intoxication
During each of several post-drinking assessments, participants in the placebo and alcohol conditions rated their feelings of intoxication by responding to the item, “How drunk do you feel right now?” using a scale ranging from 0 (not drunk at all) to 10 (more drunk than I have ever been). At the end of the study, these participants also indicated the number of standard drink equivalents they believed they had consumed using an open-ended response item.
Electrophysiological recording
The electroencephalogram (EEG) was recorded continuously throughout the experimental task from 32 tin electrodes fixed in a stretch-lycra cap (ElectroCap, Eaton, OH) placed on the scalp in standard locations (Electrode Position Nomenclature Committee, 1994) and referenced to the right mastoid; an average mastoid reference was derived offline. The EEG signal was amplified with a Synamps2 amplifier (Compumedics Neuroscan, Charlotte, NC), filtered on-line at 0.05 to 40 Hz and sampled at 1000 Hz. Electrode locations were cleaned until the measured impedance of the skin was below 5 kΩ. Ocular artifacts (blinks) were removed from the EEG off-line using a regression-based procedure (Semlitsch, Anderer, Schuster, & Presslich, 1986). EEG data were segmented into epochs of −100 to 1200 ms of post-stimulus activity to derive stimulus-locked ERPs, and −400 to 600 ms of post-response activity to derive response-locked ERPs. Epochs containing artifacts (e.g., muscle movement) were rejected (on the basis of visual inspection of each participant’s single-trial waveforms) prior to averaging according to participant and stimulus conditions.
Procedure
Upon arrival at the lab, participants read and signed an informed consent form, completed a number of questionnaires not relevant to this report, and were randomly assigned to one of the beverage conditions. Women self-administered a urine-stream pregnancy test (all were negative), and men also voided the bladder prior to continuing. Participants were escorted to a sound-proof recording chamber where baseline BrAC was taken, after which experimenters placed and tested recording electrodes. Next, participants completed practice trials as described previously. An experimenter (unaware of the true contents of the beverage) mixed (in view of participants) and served the beverage. After beverage consumption and the absorption period, a second BrAC was administered, along with the subjective intoxication items. Participants then completed the flanker task, stopping for a short break (~30 sec) between blocks. A longer break was given after blocks 3 and 7, during which BrAC was administered along with subjective intoxication items. After the remaining flanker trials, participants completed a few post-experimental questionnaires. Electrodes were then removed and participants were debriefed, after which control and placebo participants were dismissed. Alcohol group participants remained in the lab until a breathalyzer test indicated that they were sober (BrAC ≤ .02%).
Results
Manipulation Check
Baseline BrAC was 0.0 in all participants and remained that way throughout the experiment for control and placebo participants. For alcohol participants, BrAC increased throughout most of the task (pretask M = .073%; after block 3 M = .078%; after block 7 M = .082%), F(2, 64) = 3.11, p = .05, before stabilizing (immediate post-task M = .081%), t(32) = .024, p = .81. Alcohol group participants reported feeling more intoxicated throughout the study (M = 4.27) than placebo group participants (M = 2.30), F(1, 62) = 39.88, p < .001. However, the pattern of subjective intoxication responses across assessments [increasing from pretask to midtask and decreasing thereafter; F(2,118) = 6.21, p = .01] did not vary in the placebo and alcohol conditions (Group × Time interaction: F < 1). Post-experiment estimates of the number of standard drinks consumed were higher in the alcohol group (M = 4.31) than the placebo group (M = 2.13), F(1, 62) = 41.50, p < .001. That placebo participants believed they had consumed approximately 2 standard drinks on average supports the effectiveness of the placebo manipulation.
Behavioral Data
Due to computer errors, data from three participants in the control group were excluded from the analyses. RTs were limited to responses made between 100 ms and 1500 ms after target onset, to reduce the influence of a few outlying data points (< 1% of trials) and to eliminate fast “guessing” responses. Due to the relatively small number of trials contributing to post-error responses, within-group variance for these trials remained high. To reduce this within-group variability, the mean and standard deviation for post-error trials was calculated and a 1.5 SD cut-off was used to define outliers. A Winsorization scheme was used to modify the outlying data points by changing their values to the next most-extreme, non-outlying value in the distribution, maintaining their ordinal position while reducing their influence on the mean (see Tabachnick & Fidell, 2013; Wilcox, 2012). Nine data points were changed across the three groups. Accuracy data (proportion correct) were transformed using arcsine of the square root, which normalizes variance across conditions to produce distributions more suitable for analysis of variance (ANOVA). However, for ease of interpretation, raw (untransformed) means are presented in the text and Table 1. Accuracy and RT were examined in separate 3 (Group: control, placebo, alcohol) × 2 (Current trial type: compatible, incompatible) × 2 (Previous trial accuracy: correct, incorrect) mixed factorial ANOVAs with repeated measures on the latter factors.
Table 1.
Mean Accuracy and RT by Group, Previous Trial Accuracy, and Current Trial Compatibility
| Current trial | |||||||
|---|---|---|---|---|---|---|---|
| Accuracy | RT | ||||||
| Previous trial accuracy |
Comp | Incomp | F | Comp | Incomp | F | |
| Control | |||||||
| Correct | 0.97 | 0.91 | 92.94** | 371 | 422 | 254.86** | |
| 0.03 | 0.06 | 68 | 69 | ||||
| Incorrect | 0.96 | 0.91 | 4.30* | 426 | 473 | 26.01** | |
| 0.06 | 0.09 | 94 | 88 | ||||
| Placebo | |||||||
| Correct | 0.99 | 0.90 | 178.17** | 374 | 432 | 300.79** | |
| 0.01 | 0.07 | 76 | 82 | ||||
| Incorrect | 0.97 | 0.93 | 8.80* | 412 | 456 | 28.17** | |
| 0.06 | 0.09 | 90 | 85 | ||||
| Alcohol | |||||||
| Correct | 0.97 | 0.88 | 127.99** | 377 | 427 | 200.31** | |
| 0.02 | 0.07 | 70 | 72 | ||||
| Incorrect | 0.96 | 0.90 | 10.72** | 437 | 503 | 131.14** | |
| 0.06 | 0.09 | 109 | 119 | ||||
Note. Comp = compatible arrays; Incomp = incompatible arrays. Italicized numbers are standard deviations. Columns labeled “F” provide values of F-tests comparing compatible versus incompatible means for each condition.
p < .05;
p < .01.
Accuracy
The main effect of Current trial type was significant, F(1, 90) = 113.20, p < .001, with accuracy being greater for compatible (M = 0.97, SD = 0.04) than incompatible (M = 0.90, SD = 0.08) trials. The main effect of Previous trial accuracy also was significant, F(1, 90) = 17.11, p < .001, with accuracy being greater following incorrect responses (M = 0.94, SD = 0.08) than following correct responses (M = 0.93, SD = 0.07). These effects were qualified by a significant Current trial type × Previous trial accuracy interaction, F(1, 90) = 11.35, p = .001. Planned comparisons revealed that accuracy on compatible trials did not differ significantly following correct (M = 0.98, SD = 0.02) and incorrect (M = 0.96, SD = 0.06) responses, F < 1.0, p > .05. For incompatible trials, however, accuracy was greater following incorrect responses (M = 0.91, SD = 0.09) than following correct responses (M = 0.89, SD = 0.07), F(1, 90) = 22.69, p < .001. The main effect and interactions with Group were not significant, Fs < 1, ps > .35.
RT
The main effect of Current trial type was significant, F(1, 90) = 397.97, p < .001, with incompatible trials eliciting slower responses (M = 452 ms, SD = 83) than compatible trials (M = 399 ms, SD = 81). The main effect of Previous trial accuracy also was significant, F(1, 90) = 93.93, p < .001, revealing slower response times following errors (M = 451 ms, SD = 97) than correct trials (M = 401 ms, SD = 72).
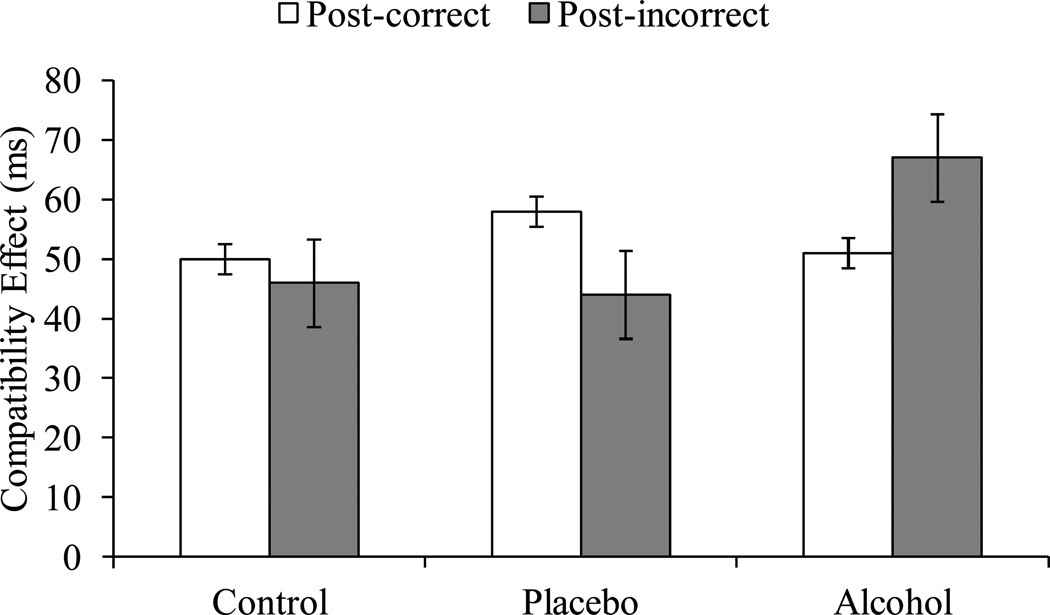
The Group × Previous trial accuracy interaction was significant, F(2, 90) = 4.58, p = .01, and was qualified by a significant Group × Current trial type × Previous trial accuracy interaction, F(2, 90) = 4.26, p = .02 (Table 1). To simplify display and interpretation of this interaction, and for comparability with previous reports (see Bartholow et al., 2012; Ridderinkhof et al., 2002), we subtracted compatible trial RTs from incompatible trial RTs to create a CE for each participant. Figure 1 displays the CE as a function of group and previous trial accuracy. For the control group, the CE did not differ following errors (M = 46 ms, SD = 47) and correct responses (M = 50 ms, SD = 16), (F < 1). For the placebo group, the CE was marginally smaller on trials following errors (M = 44 ms, SD = 48) than on trials following correct responses (M = 58 ms, SD = 19),, F(1, 32) = 3.44, p = .07. In contrast, for the alcohol group, the CE was larger on trials following errors (M = 67 ms, SD = 33) than on trials following correct responses (M = 51 ms, SD = 21), F(1, 32) = 5.08, p = .03.
Figure 1.
The compatibility effect in RTs following errors and correct responses as a function of beverage group. Vertical bars represent + 1 SE.
To determine whether the beverage effect on the post-error CE was pharmacological and not due to expectancy effects, planned contrasts were conducted comparing the alcohol group with the average of the control and placebo groups (pharmacology) and the control group with the average of the placebo and alcohol groups (expectancy). The CE following errors was significantly larger in the alcohol group (M = 67 ms, SD = 33) compared to the average of the control and placebo groups (M = 45 ms, SD = 47), t(91) = 2.35, p = .02, indicating a significant pharmacological effect. However, the control group’s post-error CE (M = 46 ms, SD = 47) did not differ reliably from the average of the placebo and alcohol groups (M = 55 ms, SD = 42), t < 1, p = .36.
Confidence Ratings
Although response confidence ratings were not directly relevant to the current hypotheses, making these ratings introduced a delay between trials, raising the possibility that group differences in post-error and post-correct indices of control could arise from differences in the accuracy or the time taken to make the ratings. Across groups, participants were equally able to classify the accuracy of their responses (Control: M = .95, SD = .04; Placebo: M = .95, SD = .05; Alcohol: M = .96, SD = .02; F[2, 90] = .20, p = .82). However, the control group made these ratings more quickly (M = 2673 ms, SD = 369) than either the placebo (M = 2905 ms, SD = 293; F[1, 57] = 7.25, p = .01) or alcohol groups (M = 3029 ms, SD = 294; F[1, 57] = 17.06, p < .001), whose confidence rating RTs did not differ reliably, F(1, 62) = 2.87, p = .10. RT was included as a covariate in the ERP analyses, and was not significant, nor did its inclusion alter any effects of interest.1 Therefore, we do not consider this issue further.
ERP Data
Use of univariate repeated-measures ANOVA is commonplace in ERP research, but has several shortcomings limiting its applicability (see Vasey & Thayer, 1987). Psychophysiological data frequently violates the assumption of sphericity (i.e., that the variances of differences between factor levels are equal; Jennings, Wood, & Lawrence, 1976), and corrections (e.g., Greenhouse-Geisser or Huynh-Feldt p-value adjustments) result in loss of statistical power. Inter-individual variability in both baseline and stimulus-elicited EEG activity often is greater than variability attributable to manipulated factors of interest (see Gratton, 2000), contributing to inflated error variance estimates in ANOVA that also reduce power. Scholars have therefore advised the use of multivariate approaches for psychophysiological data (see Gratton, 2000; Vasey & Thayer, 1987), such as multilevel modeling. Advantages of multilevel modeling include relaxed assumptions concerning sphericity, the ability to simultaneously estimate both within-participant and between-participants effects (see Bryk & Raudenbush, 1992), and the ability to specify separate error terms at each level of nesting. Multilevel modeling is robust to missing observations, whereas repeated-measures ANOVA requires that missing values be interpolated or aggregated across, or that the subject’s data be discarded. Thus, assuming reasonably large samples (n > 10 + k) this approach generally yields greater power than ANOVA (Baguley, 2004). Based on these considerations, the current data were analyzed with hierarchical linear modeling (HLM) using SAS PROC MIXED (see Bryk & Raudenbush, 1992).2 Measurements of voltage at each electrode site for each condition were nested within subjects. Nuisance variance between subjects was modeled by including a random intercept of subject.
EEG data from six participants were excluded from the ERN analysis due to excessive artifact, and therefore the final sample consisted of 90 participants (control: n = 27; placebo: n = 31; alcohol: n = 32). Initial analyses indicated that the ERN emerged between 10 and 110 ms post-response and was largest over fronto-central and central scalp locations, consistent with previous research (Bartholow et al., 2012; Yeung et al., 2004). Analysis of this component was based on mean amplitudes measured 10–110 ms following the response at electrodes FC3, FCz, FC4, C3, Cz, and C4. Previous research has shown that ERN amplitude stabilizes when at least 6 error trials are included in each participant’s average waveform (Olvett & Hajcak, 2009). Due to a number of participants making fewer than 6 errors on compatible trials, analysis of the ERN was limited to incompatible trials only. Data from an additional four participants were excluded from the N2 and FSW analysis due to excessive artifact in the stimulus-locked data; therefore the final sample for these analyses consisted of 86 participants (control: n = 24; placebo and alcohol: n = 31 per group). Initial analyses showed that the amplitude of the N2 peaked between 215 to 345 ms post-stimulus onset and was most pronounced over frontal and frontal-central scalp locations, consistent with past research (van Veen & Carter, 2002). Preliminary analyses indicated the FSW emerged 800–1200 ms post-stimulus onset and was most pronounced over frontal and fronto-central electrode locations, as in previous research (see Bailey et al., 2010; West & Bailey, 2012). Analyses of the N2 and FSW included data from electrodes at frontal (F3, Fz, F4) and fronto-central (FC3, FCz, FC4) locations. Mean N2 and FSW amplitudes were submitted to separate 3 (Group: control, placebo, alcohol) × 2 (Previous trial accuracy: correct, incorrect) × 2 (Current trial type: compatible, incompatible) × 2 (Coronal: frontal, frontal-central) × 3 (Sagittal: left, midline, right) HLMs. Mean amplitudes as a function of beverage group, previous trial accuracy, and compatibility are displayed in Table 2, and Figures 3 and 4 display stimulus-locked waveforms in which the N2 and FSW are indicated. Note that because all ERP components examined here represent negative voltage deflections, larger magnitude is associated with more negative amplitude values.
Table 2.
Mean Amplitude (µV) of the N2 and FSW by Group, Previous or 2-back Trial Accuracy, and Current Trial Compatibility
| Current trial | |||||||
|---|---|---|---|---|---|---|---|
| N2 | FSW | ||||||
| Previous trial accuracy |
Comp | Incomp | t | Comp | Incomp | t | |
| Control | |||||||
| Correct | 1.48 | 0.47 | 3.78** | −2.10 | −2.70 | 1.66 | |
| 2.27 | 2.59 | 2.59 | 2.55 | ||||
| Incorrect | 2.29 | 0.82 | 5.48** | −0.29 | 0.51 | −2.25* | |
| 3.28 | 3.43 | 4.27 | 4.12 | ||||
| Placebo | |||||||
| Correct | 1.87 | 0.87 | 4.25** | −1.33 | −2.14 | 2.58** | |
| 3.73 | 3.87 | 2.94 | 3.13 | ||||
| Incorrect | 1.97 | 0.45 | 6.48** | −0.16 | −1.86 | 5.44** | |
| 4.46 | 5.05 | 3.74 | 5.44 | ||||
| Alcohol | |||||||
| Correct | 0.34 | −0.66 | 4.25** | −1.29 | −2.14 | 2.73** | |
| 2.28 | 2.54 | 2.78 | 3.01 | ||||
| Incorrect | 0.47 | 0.68 | −0.91 | 0.74 | 1.09 | −1.11 | |
| 3.06 | 3.91 | 4.60 | 4.54 | ||||
| Two-back trial accuracy | Comp | Incomp | t | Comp | Incomp | t | |
| Control | |||||||
| Correct | 1.45 | 0.46 | 3.59** | −2.18 | −2.74 | 1.64 | |
| 2.31 | 2.61 | 2.57 | 2.60 | ||||
| Incorrect | 1.30 | 0.21 | 3.94** | −0.83 | −2.96 | 6.21** | |
| 2.65 | 2.96 | 5.61 | 4.71 | ||||
| Placebo | |||||||
| Correct | 1.85 | 0.86 | 4.06** | −1.34 | −2.10 | 2.46** | |
| 3.70 | 3.90 | 2.39 | 2.71 | ||||
| Incorrect | 1.42 | 0.57 | 3.41** | −0.04 | −1.31 | 4.06** | |
| 5.78 | 4.87 | 3.59 | 4.69 | ||||
| Alcohol | |||||||
| Correct | 0.24 | −0.72 | 3.94** | −1.41 | −2.25 | 2.79** | |
| 2.35 | 2.58 | 2.73 | 2.98 | ||||
| Incorrect | 0.90 | −0.30 | 4.92** | 0.74 | −0.78 | 5.07** | |
| 3.82 | 3.65 | 4.68 | 4.36 | ||||
Note. Comp = compatible arrays; Incomp = incompatible arrays. Italicized numbers are standard deviations. Columns labeled “t” provide values of t-tests comparing compatible versus incompatible means for each condition.
p < .05;
p < .01.
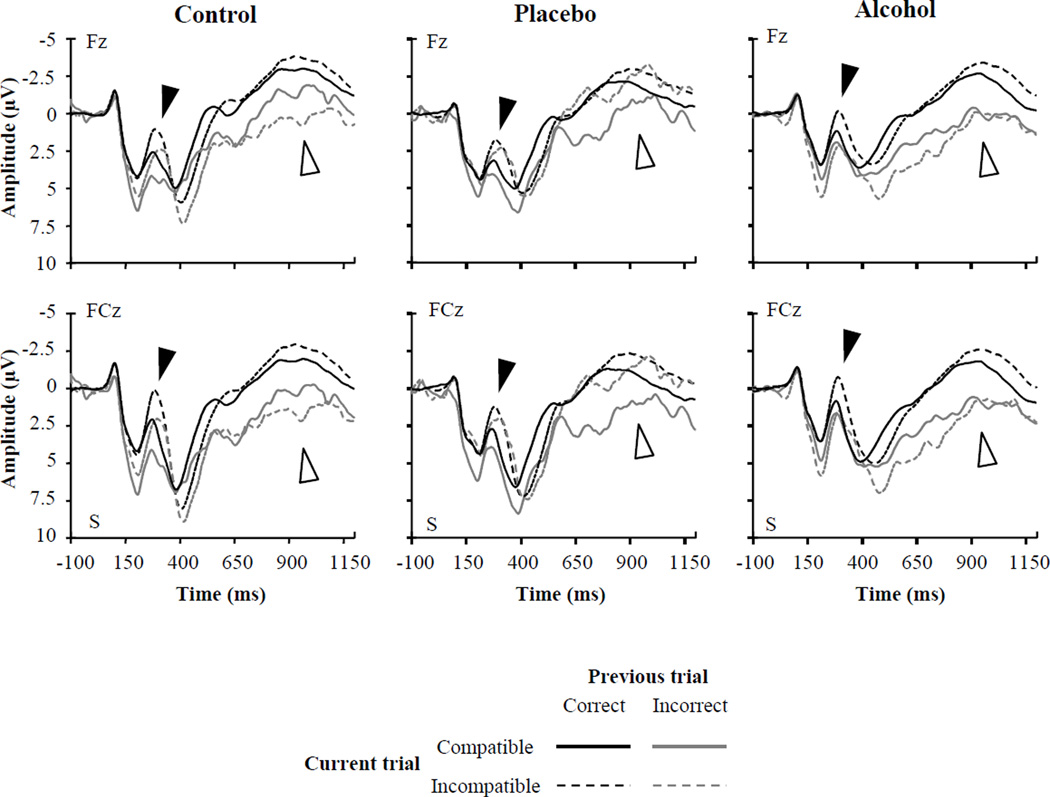
Figure 3.
Stimulus-locked ERP waveforms elicited by compatible and incompatible flanker arrays following correct and incorrect responses at frontal and fronto-central electrodes as a function of beverage group. ‘S’ (time zero) indicates stimulus array onset. The N2 is visible as the prominent negative peak at approximately 300 ms post-stimulus (black arrows), and the FSW is visible as the negativity between approximately 800 to 1200 ms post-stimulus onset (white arrows).
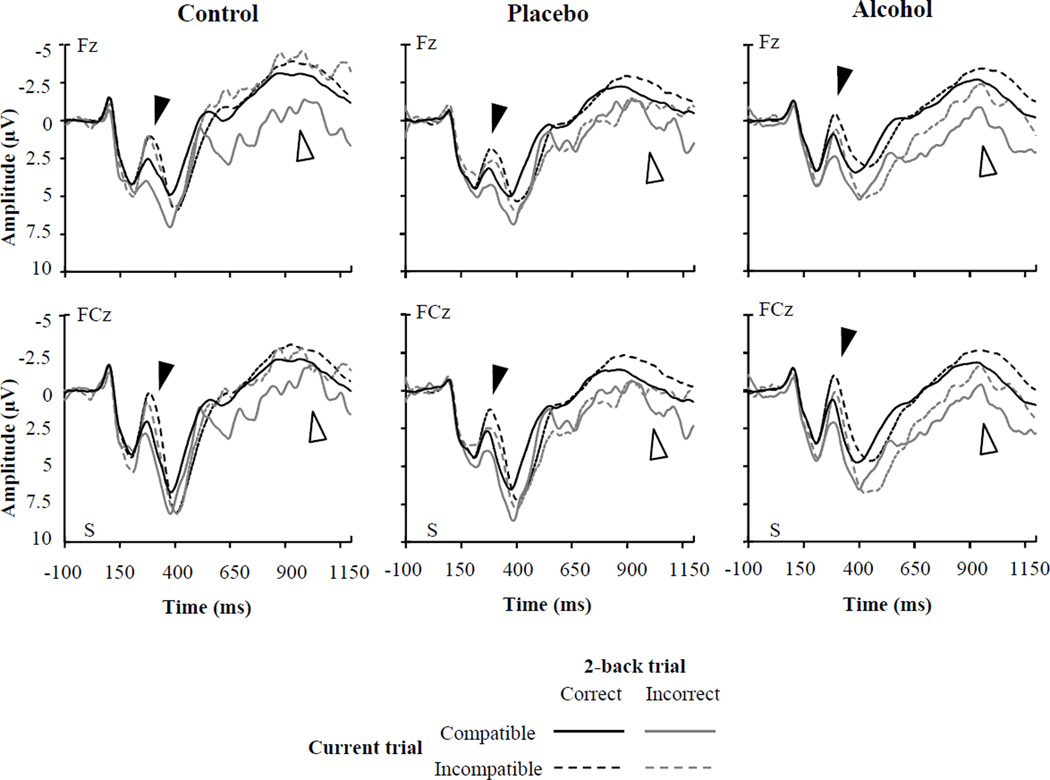
Figure 4.
Stimulus-locked ERP waveforms elicited by compatible and incompatible flanker arrays two trials following correct and incorrect responses at frontal and fronto-central electrodes a function of beverage group. ‘S’ (time zero) indicates stimulus onset. The N2 is visible as the prominent negative peak at approximately 300 ms post-stimulus (black arrows), and the FSW is visible as the negativity between approximately 800 to 1200 ms post-stimulus onset (white arrows).
ERN
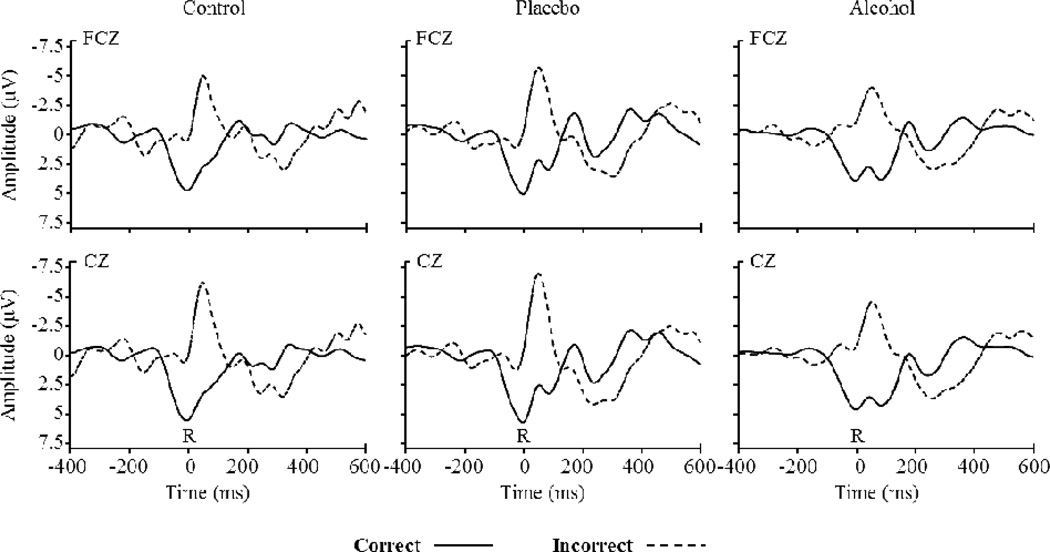
ERN amplitude on incompatible trials was submitted to a 3 (Group: control, placebo, alcohol) × 2 (Accuracy: correct, incorrect) × 2 (Coronal: frontal-central, central) × 3 (Sagital: left, midline, right) HLM. A main effect of Accuracy, F(1, 957) = 1099.68, p < .001, with greater amplitude on incorrect (M = −5.08 µV) than correct (M = 1.14 µV) trials, was qualified by a Group × Accuracy interaction, F(2, 957) = 3.34, p = .04. ERN amplitude was greater for incorrect trials in the placebo group (M = −5.88 µV) compared to the alcohol group (M = −4.14 µV), t(957) = 2.54, p = .01, consistent with previous work (Bartholow et al., 2012; Ridderinkhof et al., 2002). ERN amplitude was marginally greater in the control group (M = −5.20 µV) compared to the alcohol group, t(957) = 1.49, p = .13, and did not differ significantly between the control and placebo groups, t < 1, p = .34. For correct trials, amplitude was marginally greater for the placebo group (M = .93 µV) compared to the alcohol group (M = 2.09 µV), t(957) = 1.70, p = .08, and was greater in the control group (M = .40 µV) compared to the alcohol group, t(957) = 2.38, p = .02; control and placebo groups’ amplitudes did not differ, t < 1, p = .46.
Planned contrasts to test for pharmacological versus expectancy effects showed that ERN amplitude for errors was significantly smaller in alcohol group (M = −4.14 µV) compared to the average of control and placebo groups (M = −5.56 µV), t(957) = 2.34, p = .02, consistent with a pharmacological effect. The control group (M = −5.20 µV) did not differ reliably from the average of placebo and alcohol groups (M = −4.99 µV), t < 1, p = .76.
N2
The analysis showed significant effects of Current trial type, F(1, 1969) = 91.78, p < .0001, indicating larger amplitudes on incompatible (M = 0.44 µV) compared to compatible trials (M = 1.40 µV), and Previous trial accuracy, F(1, 1969) = 14.57, p < .001, indicating larger amplitudes following correct (M = 0.73 µV) compared to incorrect responses (M = 1.11 µV). These effects were qualified by a Group × Previous trial accuracy × Current trial type interaction, F(2, 1969) = 8.43, p < .001 (see Table 2). On trials following correct responses incompatible arrays elicited larger N2s than compatible arrays for participants in all beverage groups. However, on trials following errors, this pattern was evident in the placebo and control groups only.
Planned contrasts showed that the CE in N2 amplitude was significantly smaller in alcohol group (M = 0.21 µV) compared to the average of the control and placebo groups (M = 1.5 µV), t(1969) = −5.79, p <.001, consistent with a pharmacological effect.. The N2 CE was significantly greater in the control group (M = 1.47 µV) compared to the average of the placebo and alcohol groups (M = 0.66µV), t(1969) = 2.57, p = .01, consistent with an expectancy effect.
To determine whether conflict monitoring had rebounded in the alcohol group on subsequent trials, N2 amplitude elicited by compatible and incompatible arrays occurring two trials following both errors and correct responses (i.e., 2-back accuracy) was analyzed using an additional HLM. The effect of Current trial type was significant, F(1, 1957) = 94.07, p < .0001, indicating larger amplitudes overall for incompatible trials (M = 0.18 µV) than compatible trials (M = 1.20 µV). The Group × 2-back accuracy interaction was significant, F(2, 1957) = 7.62, p < .001, indicating that the N2 was larger overall 2 trials following an error than 2 trials following a correct response for the placebo group (Ms = 0.99 and 1.36 µV, respectively; t[1957] = 2.05, p < .05), but not for the control group (Ms = 0.75 and 0.96 µV, respectively; t[1957] = 1.04, p = .30) or the alcohol group, for whom the N2 was smaller 2 trials following errors (M = 0.30 µV) than 2 trials following correct responses (M = −0.24 µV), t[1957] = −3.15, p < .01. Critically, the Group × 2-back accuracy × Current trial type interaction was not significant (F < 1). As indicated in the bottom portion of Table 2 (and see Figure 4), for participants in all beverage groups incompatible arrays elicited larger N2s than compatible arrays two trials following correct responses and errors.
FSW
Significant main effects of Previous trial accuracy, F(1, 1969) = 213. 18, p < .001, indicating larger amplitudes following correct responses (M = −1.95 µV) than incorrect responses (M = 0.01 µV) and Current trial type, F(1, 1969) = 12.25, p < .001, indicating larger amplitudes overall on incompatible trials (M = −1.21 µV) than on compatible trials (M = −0.73 µV), were qualified by a Group × Previous trial accuracy × Compatibility interaction, F(2, 1969) = 7.80, p < .001. Planned contrasts showed that, on trials following correct responses, incompatible arrays elicited larger FSW amplitude than compatible arrays for participants in all beverage groups (this effect was marginal in the control group), consistent with the N2 data. However, on trials following errors, only placebo group participants showed a larger FSW on incompatible than compatible trials.
Additional contrasts indicated that the CE in FSW amplitude was significantly smaller in alcohol group (M = −0.35 µV) compared to the average of the control and placebo groups (M = 0.56 µV), t(1969) = −2.04, p = .04, and that the CE in FSW amplitude was significantly smaller in the control group (M = 0.80 µV) compared to the average of the placebo and alcohol groups (M = −0.63 µV), t(1969) = −3.52, p < .001, indicating both pharmacological and expectancy effects on the FSW.
To determine whether performance adjustment had rebounded in the alcohol and control groups on subsequent trials, we compared FSW amplitudes elicited by compatible and incompatible trials occurring two trials following errors and correct responses (i.e., 2-back accuracy) using an additional HLM. The waveforms depicted in Figure 4 appear to indicate that participants in all groups showed FSW compatibility effects by the second trial following errors. Current trial type was significant, F(1, 1923) = 82.28, p < .0001, indicating larger amplitudes overall on incompatible trials (M = −2.03 µV) than on compatible trials (M = −.84 µV). The Group × 2-back accuracy interaction also was significant, F(2, 1923) = 7.80, p < .001, indicating that the FSW was larger 2 trials following correct responses than 2 trials following errors in all groups, but this effect was especially large in the alcohol group (Ms = −1.83 & −0.02 µV, respectively), t(1923) = −8.48, p < .001. The effect was less pronounced for those in the placebo (Ms = −1.72 & −0.68 µV, respectively), t(1923) = −4.73, p < .001, and control groups (Ms = −2.46 & −1.89 µV, respectively), t(1923) = −2.32, p < .05. Critically, the Group × 2-back accuracy × Current trial type interaction was not significant, F(2, 1923) = 1.48, p = .23. Planned comparisons confirmed that incompatible arrays elicited larger FSW amplitude than compatible arrays two trials following correct responses and errors in all groups (see Table 2, bottom).
Associations of Neural and Behavioral Indices of Control
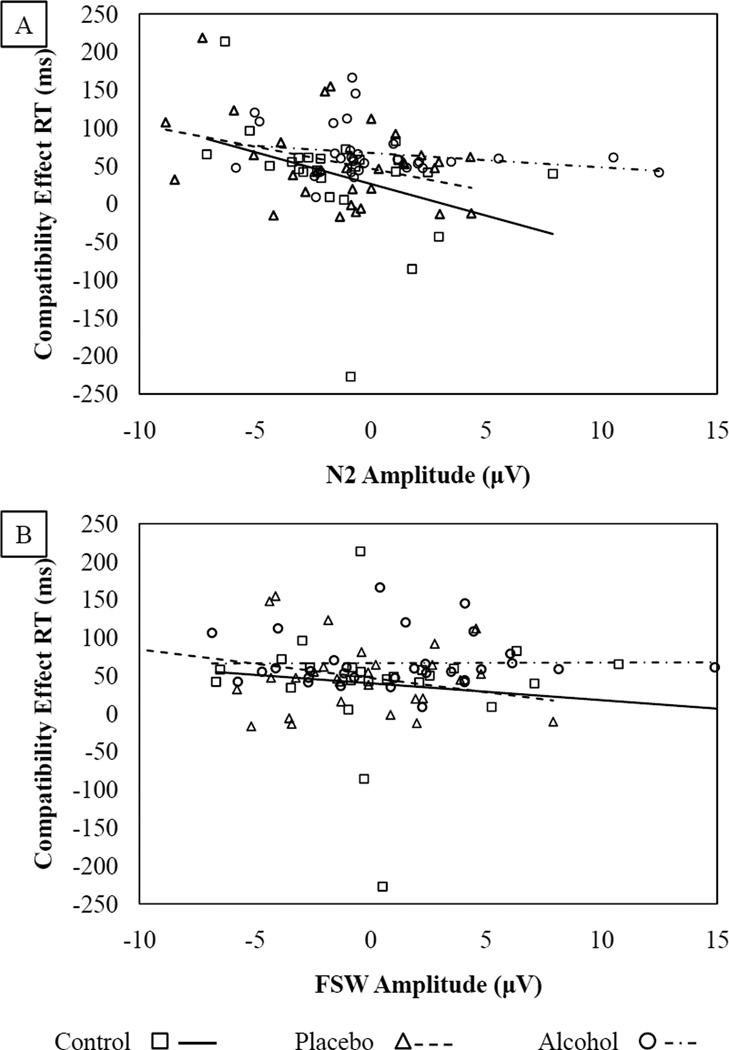
In a previous study examining alcohol effects on links between error processing and a behavioral manifestation of control (see Jacoby, 1991), placebo and control group participants’ ERN responses were significantly associated with control estimates but alcohol participants’ responses were not (Bartholow et al., 2012), suggesting that alcohol disrupts the typical networks through which neural processes exert control over behavior. To test whether similar patterns might emerge with other control indices, we computed separate linear regressions in which the CE in RT (behavioral control) was regressed on the CE in the N2 and FSW following incorrect responses (neural control), beverage group, and their respective interactions. Scatterplots depicting these associations are shown in Figure 5.
Figure 5.
Scatterplots showing the relationship between the post-error CE in the RTs and the amplitude of the N2 (panel A) and FSW (panel B). Amplitude reflects incompatible – compatible trials post-errors.
The Beverage group × N2 interaction significantly predicted the CE in RT, β = −.38, t(83) = −3.62, p = .001. This interaction was probed by computing separate linear regressions testing the association between N2 and RT CEs in each beverage group. N2 amplitude predicted the size of the CE in RT for those in the placebo group, β = −.53, t(29) = −3.39, p = .002, and (marginally) the control group, β = −.36, t(22) = −1.83, p = .08. This was not the case for the alcohol group, however, β = −.21, t(29) = −1.18, p = .25 (see Figure 5A). The full model explained a significant proportion of variance in the CE, R2 = .15, F(2, 83) = 7.02, p = .002.
The Beverage group × FSW interaction was a significant predictor of the CE in RT, β = −.24, t(83) = −2.17, p = .03. For the placebo group, FSW amplitude was a significant predictor of the size of the CE in RT, β = −.37, t(29) = −2.11, p = .04. This was not the case for the control or alcohol groups, β = −.02, t < 1, p = .94 and β = .01, t < 1, p = .96, respectively (see Figure 5B). The full model explained a marginally significant proportion of variance in the CE, R2 = .06, F(2, 83) = 2.78, p = .06.
Discussion
The goal of the current study was to clarify alcohol’s effects on cognitive control by determining whether impairment of two crucial components of adaptive functioning—conflict monitoring and performance adjustment—is limited to situations in which control failures have occurred. Better understanding of alcohol’s acute effects on these basic cognitive processes and their neural underpinnings has the potential to inform interventions aimed at curbing a number of problem behaviors, including so-called “loss-of-control” drinking (e.g., Field et al., 2010; see also de Wit, 1996) and risk-related decisions in a number of domains (e.g., Giancola, 2000; Fromme et al., 1997; MacDonald et al., 1995, 1996). We predicted that conflict monitoring and performance adjustment would be disrupted by alcohol on trials following an error (i.e., when control has failed), but not on trials following correct responses (i.e., when control was adequate). We hypothesized that alcohol’s impairment of these processes can be characterized in terms of delayed recovery of control, and predicted that the alcohol group would show neural evidence of resumed conflict monitoring and performance adjustment two trials after an error.
Consistent with these hypotheses, the data showed that neural processes supporting conflict monitoring and performance adjustment were intact in the alcohol group following correct responses but were attenuated during and following errors. Relative to placebo and control conditions, alcohol attenuated the amplitude of the ERN and disrupted post-error behavioral adjustments, consistent with previous research (Bartholow et al., 2012; Ridderinkhof et al., 2002). Additionally and unique to this study, the N2 and FSW, reflecting neural activity in medial (i.e., ACC) and dorsolateral areas of prefrontal cortex believed to support conflict monitoring and performance adjustment, respectively (see Botvinick et al., 2004; Kerns et al., 2004; McGuire & Botvinick, 2010), were essentially absent on trials immediately following errors in the alcohol group, but returned two trials following errors. We are the first to demonstrate such a pattern, providing evidence that alcohol’s effects on cognitive control, at least at the typical laboratory dose tested here, appear largely restricted to recovery from control failures.
The current finding that alcohol reduced the CE in the amplitude of the N2 might seem inconsistent with previous reports showing no effect of alcohol on N2 amplitude (Easdon et al., 2005; Ridderinkhof et al., 2002; Rohrbaugh et al., 1987). However, those prior reports are perfectly in-line with the current finding that alcohol has no effect on conflict monitoring on trials that follow correct responses. Given that the majority of the trials in any dataset like this will represent neural activity following correct responses, and that none of those prior studies (or, for that matter, any prior studies of alcohol effects on ERPs) examined neural responses separately as a function of previous trial accuracy, it is not surprising that previous work has failed to find effects of alcohol on N2 amplitude.
Taken together, the behavioral and neurophysiological data from this study point to a sequence of events set in motion by errors that differentiate intoxicated from sober control performance. According to several theoretical models (Botvinick et al., 2001, 2004; Braver, 2012; Holroyd & Coles, 2002; Yeung et al., 2004), transient performance fluctuations are tracked by areas of medial prefrontal cortex, such as the ACC. Performance that does not meet current goals triggers activation of the ACC and neighboring structures, reflected in the amplitude of the ERN, ostensibly to alert other areas of prefrontal cortex that increased control is needed to maintain desired performance. Following alcohol consumption this alert is attenuated, which in theory results in inadequate signaling of neural structures involved in control implementation. The current data suggest that this inadequate signaling has implications for both conflict monitoring and performance adjustment processes, reflected in the amplitude of the N2 and FSW, respectively, and for behavioral performance as reflected in the magnitude of the response time CE. Indeed, the analyses showing that interactions between neural responses (especially the N2) and beverage group contribute to prediction of the response time CE suggest a functional role for these neural processes in producing behavioral manifestations of control. The fact that these associations appear broken in the alcohol group suggests that alcohol disrupts the neural networks typically linking activity in the ACC and other areas of prefrontal cortex with behavioral control (see also Bartholow et al., 2012; Ridderinkhof et al., 2002).
The current data also suggest that expectancy or motivational processes might play a role in post-error implementation of control, particularly in the performance adjustment process. Specifically, the control group did not show a typical compatibility effect in the FSW on post-error trials, and although planned contrasts indicated significant expectancy as well as pharmacological effects in both the N2 and FSW components, the control group’s post-error FSW responses were more similar to those of the alcohol group than the placebo group, suggesting the possibility that this aspect of cognitive control is more sensitive to expectancy-related than to pharmacological effects of alcohol. Findings from a number of recent studies are consistent with the idea that participants who consume a placebo engage greater cognitive resources and (sometimes) behaviorally outperform those in the control group (e.g., Bartholow et al., 2012; Fillmore, Mulvihill, & Vogel-Sprott, 1994; Fillmore & Vogel-Sprott, 1995; Saults, Cowan, Sher, & Moreno, 2007; Williams, Goldman, & Williams, 1981). Several theorists have posited that this effect stems from placebo participants anticipating cognitive impairment from alcohol and therefore exerting more effort during cognitive tasks compared to control participants (e.g., Fillmore & Blackburn, 2002; Marczinski & Fillmore, 2005; Williams et al., 1981).
Theoretical accounts from a number of perspectives have long posited a strong link between motivation and cognitive control (e.g., Kruglanski, Shah, Fishbach, Friedman, & Chun, 2002; Simon, 1967), and recent findings from cognitive neuroscience have identified specific patterns of neural activation supporting this link (see Chiew & Braver, 2011). For example, data from recent fMRI studies examining cognitive control across a range of task domains have confirmed earlier single-unit recording studies in primates (Leon & Shadlen, 1999; Watanabe, 1996) indicating that specific regions within lateral prefrontal cortex (and some associated regions, including the ACC) are highly sensitive to the interaction of task demands and motivational value (see Jimura, Locke, & Braver, 2010; Kouneiher et al., 2009; Locke & Braver, 2008; Taylor et al., 2004). These same structures have been implicated in the generation of the FSW (West & Bailey, 2012; West et al., 2012). Thus, the current findings draw a connection between previous work on alcohol expectancy effects on cognition (e.g., Fillmore & Blackburn, 2002; Marczinski & Fillmore, 2005; Williams et al., 1981) and brain imaging studies on interactions between motivation and cognitive control (see Chiew & Braver. 2011), suggesting a way in which the interface of these two literatures might shed light on how and why alcohol affects cognition and performance.
Beyond differentiating effects of alcohol on post-correct versus post-error cognitive control processes, the current findings have important implications for understanding potential differences in alcohol’s effects on separable components of control outlined in recent models. In particular, Braver’s (2012; Braver, Paxton, Locke, & Barch, 2009) Dual Mechanisms of Control (DMC) model posits a dual-process framework for understanding control in which goal-directed performance is governed by the interplay of proactive control (i.e., the sustained maintenance of goal information over time that serves to bias information processing in favor of task-relevant goals) and reactive control (i.e., just-in-time conflict resolution and performance adjustment that arises from the presence of conflict).
The conflict monitoring and performance adjustment processes that were the focus of the current study are most closely aligned with the DMC notion of reactive control. That is, control failures (and, to a lesser extent, any high-conflict trials) elicit reactive efforts to reinstate control, often reflected in reduced CEs in behavior and heightened neural responses to conflict (see Bartholow et al., 2005; Gehring & Taylor, 2004; Gratton et al., 1992; Kerns et al., 2004). As seen in the current data and other recent reports (see Bartholow et al., 2012; Curtin & Fairchild, 2003; Ridderinkhof et al., 2002), all of which involved an equal probability of high- and low-conflict trials, alcohol disrupts these reactive control processes. However, alcohol appears not to have such robust effects on proactive control. As noted previously, intoxicated performance appears not to suffer under conditions in which conflict is likely or predictable (see Bartholow et al., 2003; Gustafson & Kallmen, 1990a, 1990b; Tarter et al., 1971), conditions that more strongly engage proactive than reactive control (e.g., Burgess & Braver, 2010; see also Gratton, Coles, & Donchin, 1992). Thus, one possibility suggested by the current data is that alcohol’s effects on performance, both in laboratory tasks and in behaviors occurring in natural environments, are due primarily to impairment of reactive control processes, particularly in terms of the cognitive system’s ability to process and recover from errors. Future studies employing manipulations of conflict probability or other tasks designed to differentiate the two modes of control (e.g., AX-CPT; Braver et al., 2009) will provide needed clarification on this issue.
The current study suffered from a number of limitations. First, requiring participants to make accuracy judgments following every trial complicates investigation of the hypothesis that alcohol’s impairment of control can be characterized in terms of delayed recovery following failures. This additional task added around 3 seconds to the ITI, making direct comparisons to other studies using shorter ITIs somewhat difficult. The act of making overt accuracy judgments after each trial also could have affected the control requirements of the task. However, if anything, the longer ITI and addition of accuracy ratings should have worked against finding differences in conflict monitoring and performance adjustment between the groups on post-error trials, and thus the current finding that it takes at least 2 trials (on average) following an error for intoxicated participants to recover those control functions should be considered a lower bound.
Also, the current study should be considered an initial step toward future work in which alcohol's effects on cognitive control are more directly linked to clinically-relevant outcomes, such as alcohol use disorder. The discovery that alcohol primarily impairs control-related processes once control has failed has considerable theoretical implications for understanding phenomena such as loss of control drinking (see Field, Wiers, Christiansen, Fillmore, & Verster 2010). Future studies should investigate whether individual differences in cognitive abilities or risk factors for alcohol use disorder moderate acute effects of alcohol on recovery from control failures, and whether such moderators determine potential links between laboratory effects and real-world drinking outcomes. Finally, the use of a single alcohol dose precludes examination of potentially important dose-response effects. Other work (Bartholow et al., 2003) has shown that alcohol’s effects on cognitive outcomes can vary according to dose, and it would be of interest to determine if the same is true for the post-error control processes examined here. The amount of time necessary to recover from control failures may well vary by the amount of alcohol consumed. Additionally, the sedative effects and significant motor disruption caused by higher doses of alcohol (Hindmarch, Kerr, & Sherwood, 1991) may contribute more to impaired behavior than the conflict monitoring and control adjustments examined here.
In summary, the current findings are generally consistent with considerable work indicating that alcohol negatively impacts cognitive control. The current results go beyond previous findings by specifying that such effects appear limited to processes involved in recovery from control failure, at least in situations where overall performance is similar across alcohol and no-alcohol groups and where the probability of conflict is equal. In other words, when “all is well,” a moderately intoxicating dose of alcohol appears to have little effect on neural and behavioral indices generally thought to reflect the control of goal-directed action. Once control slips—which it inevitably does for most people—the presence of alcohol results in a cascade of neurocognitive impairments that contribute to difficulty in recovering control and, most likely, increases the probability of potentially harmful impulsive, externalizing behaviors.
Figure 2.
Response-locked ERP waveforms at electrodes FCz, and Cz on incompatible trials as a function of accuracy and beverage group. ‘R’ (time zero) indicates response onset. The ERN is visible as the prominent negativity for incorrect trials peaking approximately 80 ms post-response.
Acknowledgments
Funding for this research was provided by grant R21 AA017282 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) to Bruce D. Bartholow; preparation of this manuscript was supported by grants P60 AA011998 and R01 AA020970 from the NIAAA.
Footnotes
ERN (Confidence ratings, F < 1, p = .38; Group × Previous trial accuracy, F(2, 957) = 3.34, p = .04); N2 (Confidence ratings, F < 1, p = .94; Group × Previous trial accuracy × Current trial type, F(2, 1969) = 8.43, p < .001); FSW (Confidence ratings, F(1, 1969) = 2.17, p = .14; Group × Previous trial accuracy × Current trial type, F(2, 1969) = 7.80, p < .001).
We also tested all ERP hypotheses using traditional repeated-measures ANOVAs. These models largely replicated the findings reported in the text, with the exception that the Group × Current trial type × Previous trial accuracy interaction in N2 and FSW amplitude were only marginally significant, Fs < 2.05, ps > .10.
References
- Alexander WH, Brown JW. Computational models of performance monitoring and cognitive control. Topics in Cognitive Neuroscience. 2010;2:658–677. doi: 10.1111/j.1756-8765.2010.01085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Harmon-Jones E, Devine PG, Hartley SL, Covert AE. Neural signals for the detection of unintentional race bias. Psychological Science. 2004;15:88–93. doi: 10.1111/j.0963-7214.2004.01502003.x. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Baguley T. Understanding statistical power in the context of applied research. Applied Ergonomics. 2004;35:78–80. doi: 10.1016/j.apergo.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Bailey K, West R, Anderson CA. A negative association between video game experience and proactive cognitive control. Psychophysiology. 2010;47:34–42. doi: 10.1111/j.1469-8986.2009.00925.x. [DOI] [PubMed] [Google Scholar]
- Banich MT, Mackiewicz KL, Depue BE, Whitmer AJ, Miller GA, Heller W. Cognitive control mechanisms, emotion and memory: A neural perspective with implications for psychopathology. Neuroscience & Biobehavioral Reviews. 2009;33:613–630. doi: 10.1016/j.neubiorev.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholow BD, Dickter CL, Sestir MA. Stereotype activation and control of race bias: Cognitive control of inhibition and its impairment by alcohol. Journal of Personality and Social Psychology. 2006;90:272–287. doi: 10.1037/0022-3514.90.2.272. [DOI] [PubMed] [Google Scholar]
- Bartholow BD, Henry EA, Lust SA, Saults JS, Wood PK. Alcohol effects on performance monitoring and adjustment: Affect modulation and impairment of evaluative cognitive control. Journal of Abnormal Psychology. 2012;121:173–186. doi: 10.1037/a0023664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholow BD, Pearson MA, Dickter CL, Sher KJ, Fabiani M, Gratton G. Strategic control and medial frontal negativity: Beyond errors and response conflict. Psychophysiology. 2005;42:33–42. doi: 10.1111/j.1469-8986.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- Bartholow BD, Pearson MA, Sher KJ, Wieman LC, Fabiani M, Gratton G. Effects of alcohol consumption and alcohol susceptibility on cognition: A psychophysiological examination. Biological Psychology. 2003;64:167–190. doi: 10.1016/s0301-0511(03)00108-x. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Braver TS. The variable nature of cognitive control: A dual mechanisms framework. Trends in Cognitive Neuroscience. 2012;16:106–113. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proceedings of the National Academy of Sciences. 2009;106:7351–7356. doi: 10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Hierarchical linear models: Applications and data analysis techniques. Newbury Park, CA: Sage; 1992. [Google Scholar]
- Burgess GC, Braver TS. Neural mechanisms of interference control in working memory: Effects of interference expectancy and intelligence. PLoS ONE. 2010;5:e12861. doi: 10.1371/journal.pone.0012861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burle B, Passamai C, Vidal F, Bonnet M, Hasbroucq T. Executive control in the Simon effect: An electromyographic and distributional study. Psychological Research. 2002;66:324–336. doi: 10.1007/s00426-002-0105-6. [DOI] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: An update of theory and data. Cognitive, Affective, & Behavioral Neuroscience. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Casbon TS, Curtin JJ, Lang AR, Patrick CJ. Deleterious effects of alcohol intoxication: Diminished cognitive control and its behavioral consequences. Journal of Abnormal Psychology. 2003;112:476–487. doi: 10.1037/0021-843x.112.3.476. [DOI] [PubMed] [Google Scholar]
- Cherpitel CJ. Alcohol-related injury and the emergency department: Research and policy questions for the next decade. Addiction. 2006;101:1225–1227. doi: 10.1111/j.1360-0443.2006.01567.x. [DOI] [PubMed] [Google Scholar]
- Chiew KS, Braver TS. Positive affect versus reward: Emotional and motivational influences on cognitive control. Frontiers in Psychology. 2011;2:1–10. doi: 10.3389/fpsyg.2011.00279. (article 279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles MGH, Scheffers MK, Holroyd CB. Why is there an ERN/Ne on correct trials? Response representations, stimulus-related components, and the theory of error-processing. Biological Psychology. 2001;56:173–189. doi: 10.1016/s0301-0511(01)00076-x. [DOI] [PubMed] [Google Scholar]
- Crocker LD, Heller W, Warren SL, O'Hare AJ, Infantolino ZP, Miller GA. Relationships among cognition, emotion, and motivation: Implications for intervention and neuroplasticity in psychopathology. Frontiers in Human Neuroscience. 2013;7:1–19. doi: 10.3389/fnhum.2013.00261. article 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JJ, Fairchild BA. Alcohol and cognitive control: Implications for regulation of behavior during response conflict. Journal of Abnormal Psychology. 2003;112:424–436. doi: 10.1037/0021-843x.112.3.424. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, Lang AR. Alcohol and emotion: Insights and directives from affective science. In: Rottenberg J, Johnson SL, editors. Emotion and psychopathology: Bridging affective and clinical science. Washington, DC, US: American Psychological Association; 2007. pp. 191–213. [Google Scholar]
- de Wit H. Priming effects with drugs and other reinforcers. Experimental and Clinical Psychopharmacology. 1996;4:5–10. [Google Scholar]
- Easdon C, Izenberg A, Armilio ML, Yu H, Alain C. Alcohol consumption impairs stimulus- and error-related processing during a Go/No-go task. Cognitive Brain Research. 2005;25:873–883. doi: 10.1016/j.cogbrainres.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Electrode Position Nomenclature Committee. Guideline thirteen: Guidelines for standard electrode position nomenclature. American Electroencephalographic Society. Journal of Clinical Neurophysiology. 1994;11:111–113. [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16:143–149. [Google Scholar]
- Eriksen CW, Hoffman JE. The extent of processing of noise elements during selective encoding from visual displays. Perception & Psychophysics. 1973;14:155–160. [Google Scholar]
- Field M, Wiers RW, Christiansen P, Fillmore MT, Verster JC. Acute alcohol effects on inhibitory control and implicit cognition: Implications for loss of control over drinking. Alcoholism: Clinical and Experimental Research. 2010;34:1346–1352. doi: 10.1111/j.1530-0277.2010.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Blackburn J. Compensating for alcohol-induced impairment: Alcohol expectancies and behavioral disinhibition. Journal of Studies on Alcohol and Drugs. 2002;63:237–246. doi: 10.15288/jsa.2002.63.237. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Mulvihill LE, Vogel-Sprott M. The expected drug and its expected effect interact to determine placebo responses to alcohol and caffeine. Psychopharmacology. 1994;115:383–388. doi: 10.1007/BF02245081. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Polydrug abusers display impaired discrimination reversal learning in a model of behavioural control. Journal of Psychopharmacology. 2006;20:24–32. doi: 10.1177/0269881105057000. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Expectancies about alcohol-induced motor impairment predict individual differences in responses to alcohol and placebo. Journal of Studies on Alcohol and Drugs. 1995;56:90. doi: 10.15288/jsa.1995.56.90. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Response inhibition under alcohol: Effects of cognitive and motivational conflict. Journal of Studies on Alcohol and Drugs. 2000;61:239–246. doi: 10.15288/jsa.2000.61.239. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M, Gavrilescu D. Alcohol effects on intentional behavior: Dissociating controlled and automatic influences. Experimental and Clinical Psychopharmacology. 1999;7:372–378. doi: 10.1037//1064-1297.7.4.372. [DOI] [PubMed] [Google Scholar]
- Fromme K, Katz E, D'Amico E. Effects of alcohol intoxication on the perceived consequences of risk taking. Experimental and Clinical Psychopharmacology. 1997;5:14–23. doi: 10.1037//1064-1297.5.1.14. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: Inhibition, error detection, and correction. Neuroimage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MG, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Gehring WG, Taylor SF. When the going gets tough, the cingulate gets going. Nature Neuroscience. 2004;7:1285–1287. doi: 10.1038/nn1204-1285. [DOI] [PubMed] [Google Scholar]
- George S, Rogers RD, Duka T. The acute effect of alcohol on decision making in social drinkers. Psychopharmacology. 2005;182:160–169. doi: 10.1007/s00213-005-0057-9. [DOI] [PubMed] [Google Scholar]
- Giancola PR. Evidence for dorsolateral and orbital prefrontal cortical involvement in the expression of aggressive behavior. Aggressive Behavior. 1995;21:431–450. [Google Scholar]
- Giancola PR. Executive functioning: A conceptual framework for alcohol-related aggression. Experimental and Clinical Psychopharmacology. 2000;8:576–597. doi: 10.1037//1064-1297.8.4.576. [DOI] [PubMed] [Google Scholar]
- Giancola PR. Difficult temperament, acute alcohol intoxication, and aggressive behavior. Drug and Alcohol Dependence. 2004;74:135–145. doi: 10.1016/j.drugalcdep.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Josephs RA, De Wall CN, Gunn RL. Applying the attention-allocation model to the explanation of alcohol-related aggression: Implications for prevention. Substance Use & Misuse. 2009;44:1263–1279. doi: 10.1080/10826080902960049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola PR, Zeichner A. Neuropsychological performance on tests of frontal-lobe functioning and aggressive behavior in men. Journal of Abnormal Psychology. 1994;103:832–835. doi: 10.1037//0021-843x.103.4.832. [DOI] [PubMed] [Google Scholar]
- Godlaski AJ, Giancola PR. Executive functioning, irritability, and alcohol-related aggression. Psychology of Addictive Behaviors. 2009;23:391–403. doi: 10.1037/a0016582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattan KE, Vogel-Sprott M. Maintaining intentional control of behavior under alcohol. Alcoholism: Clinical and Experimental Research. 2001;25:192–197. [PubMed] [Google Scholar]
- Gratton G. Biosignal processing. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 2nd ed. New York, NY, US: Cambridge University Press; 2000. pp. 900–923. [Google Scholar]
- Gratton G, Coles MGH, Donchin E. Optimizing the use of information: Strategic control of activation of responses. Journal of Experimental Psychology: General. 1992;121:480–506. doi: 10.1037//0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Sirevaag EJ, Eriksen CW, Donchin E. Pre- and poststimulus activation of response channels: A psychophysiological analysis. Journal of Experimental Psychology. 1988;14:331–344. doi: 10.1037//0096-1523.14.3.331. [DOI] [PubMed] [Google Scholar]
- Groman SM, James AS, Jentsch JD. Poor response inhibition: At the nexus between substance abuse and attention deficit/hyperactivity disorder. Neuroscience & Biobehavioral Reviews. 2009;33:690–698. doi: 10.1016/j.neubiorev.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson R, Kallmen H. Effects of alcohol on cognitive performance measured with Stroop’s color word test. Perceptual and Motor Skills. 1990a;71:99–105. doi: 10.2466/pms.1990.71.1.99. [DOI] [PubMed] [Google Scholar]
- Gustafson R, Kallmen H. Effects of alcohol on prolonged cognitive performance measured with Stroop’s color word test. Psychology Reports. 1990b;671:643–650. doi: 10.2466/pr0.1990.67.2.643. [DOI] [PubMed] [Google Scholar]
- Gustafson R, Kallmen H. Alcohol and the compensation hypothesis: A test with cognitive and psychomotor tasks. Perceptual and Motor Skills. 1990c;71:1367–1374. doi: 10.2466/pms.1990.71.3f.1367. [DOI] [PubMed] [Google Scholar]
- Hindmarch I, Kerr JS, Sherwood N. The effects of alcohol and other drugs on psychomotor performance and cognitive function. Alcohol and Alcoholism. 1991;26:71–79. [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Itami S, Uno H. Orbitofrontal cortex dysfunction in attention-deficit hyperactivity disorder revealed by reversal and extinction tasks. Neuroreport. 2002;13:2453–2457. doi: 10.1097/00001756-200212200-00016. [DOI] [PubMed] [Google Scholar]
- Jacoby LL. A process dissociation framework: Separating automatic from intentional uses of memory. Journal of Memory and Language. 1991;30:513–541. [Google Scholar]
- Jacoby LL, Jennings JM, Hay JF. Dissociating automatic and consciously controlled processes: Implications for diagnosis and rehabilitation of memory deficits. Basic and applied memory research: Theory in context. 1996;1:161–193. [Google Scholar]
- Jennings JR, Wood CC, Lawrence BE. Effects of graded doses of alcohol on speed-accuracy tradeoff in choice reaction time. Perception & Psychophysics. 1976;19:85–91. [Google Scholar]
- Jimura K, Locke HS, Braver TS. Prefrontal cortex mediation of cognitive enhancement in rewarding motivational contexts. Proceedings of the National Academy of Sciences of the USA. 2010;107:8871–8876. doi: 10.1073/pnas.1002007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, Macdonald AW, III, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kopp B, Rist F, Mattler U. N200 in the flanker task as a neurobehavioral tool for investigating executive control. Psychophysiology. 1996;33:282–294. doi: 10.1111/j.1469-8986.1996.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Kouneiher F, Charron S, Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nature Neuroscience. 2009;12:939–945. doi: 10.1038/nn.2321. [DOI] [PubMed] [Google Scholar]
- Kruglanski AW, Shah JY, Fishbach A, Friedman R, Chun WY. A theory of goal systems. Advances in Experimental Social Psychology. 2002;34:331–378. [Google Scholar]
- Lange JE. Alcohol’s effect on aggression identification: A two-channel theory. Psychology of Addictive Behaviors. 2002;16:47–55. doi: 10.1037//0893-164x.16.1.47. [DOI] [PubMed] [Google Scholar]
- Leon MI, Shadlen MN. Effect of expected reward magnitude on the response of neurons in the dorsolateral prefrontal cortex of the macaque. Neuron. 1999;24:415–425. doi: 10.1016/s0896-6273(00)80854-5. [DOI] [PubMed] [Google Scholar]
- Locke HS, Braver TS. Motivational influences on cognitive control: behavior, brain activation, and individual differences. Cognitive, Affective, & Behavioral Neuroscience. 2008;8:99–112. doi: 10.3758/cabn.8.1.99. [DOI] [PubMed] [Google Scholar]
- MacDonald TK, Zanna MP, Fong GT. Decision making in altered states: The effects of alcohol on attitudes toward drinking and driving. Journal of Personality and Social Psychology. 1995;68:973–985. doi: 10.1037//0022-3514.68.6.973. [DOI] [PubMed] [Google Scholar]
- MacDonald TK, Zanna MP, Fong GT. Why common sense goes out the window: Effects of alcohol on intentions to use condoms. Personality and Social Psychology Bulletin. 1996;22:763–775. [Google Scholar]
- Marczinski CA, Fillmore MT. Compensating for alcohol-induced impairment of control: Effects on inhibition and activation of behavior. Psychopharmacology. 2005;181:337–346. doi: 10.1007/s00213-005-2269-4. [DOI] [PubMed] [Google Scholar]
- McGuire JT, Botvinick MM. Prefrontal cortex, cognitive control, and the registration of decision costs. Proceedings of the National Academy of Sciences. 2010;107:7922–7926. doi: 10.1073/pnas.0910662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer DE, Kieras DE. A computational theory of executive cognitive processes and multiple-task performance: Part I. Basic mechanisms. Psychological Review. 1997;104:3–65. doi: 10.1037/0033-295x.104.1.3. [DOI] [PubMed] [Google Scholar]
- Mezzacappa E, Kindlon D, Earls F. Relations of age to cognitive and motivational elements of impulse control in boys with and without externalizing behavior problems. Journal of Abnormal Child Psychology. 1999;27:473–483. doi: 10.1023/a:1021936210844. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miner MH, Raymond N, Mueller BA, Lloyd M, Lim KL. Preliminary investigation of the impulsive and neuroanatomical characteristics of compulsive sexual behavior. Psychiatry Research. 2009;174:146–151. doi: 10.1016/j.pscychresns.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Mulvihill LE, Skilling TA, Vogel-Sprott M. Alcohol and the ability to inhibit behavior in men and women. Journal of Studies on Alcohol and Drugs. 1997;58:600–605. doi: 10.15288/jsa.1997.58.600. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The stability of error-related brain activity with increasing trials. Psychophysiology. 2009;46:957–961. doi: 10.1111/j.1469-8986.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Pihl RO, Paylan SS, Gentes-Hawn A, Hoaken PNS. Alcohol affects executive cognitive functioning differentially on the ascending versus descending limb of the blood alcohol concentration curve. Alcoholism: Clinical and Experimental Research. 2003;27:773–779. doi: 10.1097/01.ALC.0000065434.92204.A1. [DOI] [PubMed] [Google Scholar]
- Rabbitt PMA. Errors and error correction in choice-response tasks. Journal of Experimental Psychology. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Rabbitt PMA, Vyas SM. An elementary preliminary taxonomy for some errors in laboratory choice RT tasks. Acta Psychologica. 1970;33:56–76. [Google Scholar]
- Ridderinkhof KR, de Vlugt Y, Bramlage A, Spaan M, Elton M, Snel J, Band GP. Alcohol consumption impairs detection of performance errors in mediofrontal cortex. Science. 2002;298:2209–2211. doi: 10.1126/science.1076929. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rohrbaugh JW, Stapleton JM, Parasuraman R, Zubovic EA, Frowein HW, Varner JL, Adinoff B, Lane EA, Eckardt MJ, Linnoila M. Dose-related effects of ethanol on visual sustained attention and event-related potentials. Alcohol. 1987;4:293–300. doi: 10.1016/0741-8329(87)90026-7. [DOI] [PubMed] [Google Scholar]
- Romer D, Betancourt L, Giannetta JM, Brodsky NL, Farah M, Hurt H. Executive cognitive functions and impulsivity as correlates of risk taking and problem behavior in preadolescents. Neuropsychologia. 2009;47:2916–2926. doi: 10.1016/j.neuropsychologia.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saults JS, Cowan N, Sher KJ, Moreno MV. Differential effects of alcohol on working memory: distinguishing multiple processes. Experimental and Clinical Psychopharmacology. 2007;15:576. doi: 10.1037/1064-1297.15.6.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette M. Cognitive theory and research. In: Leonard KE, Blane HT, editors. Psychological theories of drinking and alcoholism. New York, NY, US: The Guilford Press; 1999. pp. 247–291. [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Scheffers MK, Coles MG. Performance monitoring in a confusing world: Error-related brain activity, judgments of response accuracy, and types of errors. Journal of Experimental Psychology: Human Perception and Performance. 2000;26:141–151. doi: 10.1037//0096-1523.26.1.141. [DOI] [PubMed] [Google Scholar]
- Shehzad Z, DeYoung CG, Kang Y, Grigorenko EL, Gray JR. Interaction of COMT val158met and externalizing behavior: Relation to prefrontal brain activity and behavioral performance. Neuroimage. 2012;60:2158–2168. doi: 10.1016/j.neuroimage.2012.01.097. [DOI] [PubMed] [Google Scholar]
- Simon H. Motivational and emotional controls of cognition. Psychological Review. 1967;74:29–39. doi: 10.1037/h0024127. [DOI] [PubMed] [Google Scholar]
- Smith GA, Brewer N. Slowness and age: Speed-accuracy mechanisms. Psychology and Aging. 1995;10:238–247. doi: 10.1037//0882-7974.10.2.238. [DOI] [PubMed] [Google Scholar]
- Steele CM, Josephs RA. Alcohol myopia: Its prized and dangerous effects. American Psychologist. 1990;45:921–933. doi: 10.1037//0003-066x.45.8.921. [DOI] [PubMed] [Google Scholar]
- Steele CM, Southwick L. Alcohol and social behavior: I. The psychology of drunken excess. Journal of Personality and Social Psychology. 1985;48:18–34. doi: 10.1037//0022-3514.48.1.18. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. Boston: Allyn and Bacon; 2013. [Google Scholar]
- Tarter RE, Jones BM, Simpson CD, Vega A. Effects of task complexity and practice on performance during acute alcohol intoxication. Perceptual and Motor Skills. 1971;33:307–318. doi: 10.2466/pms.1971.33.1.307. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Welsh RC, Wager TD, Phan KL, Fitzgerald KD, Gehring WJ. A functional neuroimaging study of motivation and executive function. NeuroImage. 2004;21:1045–1054. doi: 10.1016/j.neuroimage.2003.10.032. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. The Journal of Neuroscience. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, von Cramen DY. The role of intact frontostriatal circuits in error processing. Journal of Cognitive Neuroscience. 2006;18:651–664. doi: 10.1162/jocn.2006.18.4.651. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Vasey MW, Thayer JF. The continuing problem of false positives in repeated measures ANOVA in psychophysiology: A multivariate solution. Psychophysiology. 1987;24:479–486. doi: 10.1111/j.1469-8986.1987.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Watanabe M. Reward expectancy in primate prefrontal neurons. Nature. 1996;382:629–632. doi: 10.1038/382629a0. [DOI] [PubMed] [Google Scholar]
- Watson PE. Total body water and blood alcohol levels: Updating the fundamentals. In: Crow K, Batt R, editors. Human metabolism of alcohol: Vol. 1. Pharmacokinetics, medicolegal aspects, and general interest. Boca Raton, FL: CRC Press; 1989. pp. 41–58. [Google Scholar]
- West R, Alain C. Event-related neural activity associated with the Stroop task. Cognitive Brain Research. 1999;8:157–164. doi: 10.1016/s0926-6410(99)00017-8. [DOI] [PubMed] [Google Scholar]
- West R, Alain C. Evidence for the transient nature of a neural system supporting goal-directed action. Cerebral Cortex. 2000;10:748–752. doi: 10.1093/cercor/10.8.748. [DOI] [PubMed] [Google Scholar]
- West R, Bailey K. ERP correlates of dual mechanisms of control in the counting Stroop task. Psychophysiology. 2012;49:1309–1318. doi: 10.1111/j.1469-8986.2012.01464.x. [DOI] [PubMed] [Google Scholar]
- West R, Bailey K, Tiernan BN, Boonsuk W, Gilbert S. The temporal dynamics of medial and lateral frontal neural activity related to proactive cognitive control. Neuropsychologia. 2012;50:3450–3460. doi: 10.1016/j.neuropsychologia.2012.10.011. [DOI] [PubMed] [Google Scholar]
- West R, Travers S. Tracking the temporal dynamics of updating cognitive control: An examination of error processing. Cerebral Cortex. 2008;18:1112–1124. doi: 10.1093/cercor/bhm142. [DOI] [PubMed] [Google Scholar]
- Wilcox RR. Modern statistics for the social and behavioral sciences: A practical introduction. London, UK: Chapman & Hall/CRC; 2012. [Google Scholar]
- Williams RM, Goldman MS, Williams DL. Expectancy and pharmacological effects of alcohol on human cognitive and motor performance: The compensation for alcohol effect. Journal of Abnormal Psychology. 1981;90:267. doi: 10.1037//0021-843x.90.3.267. [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]