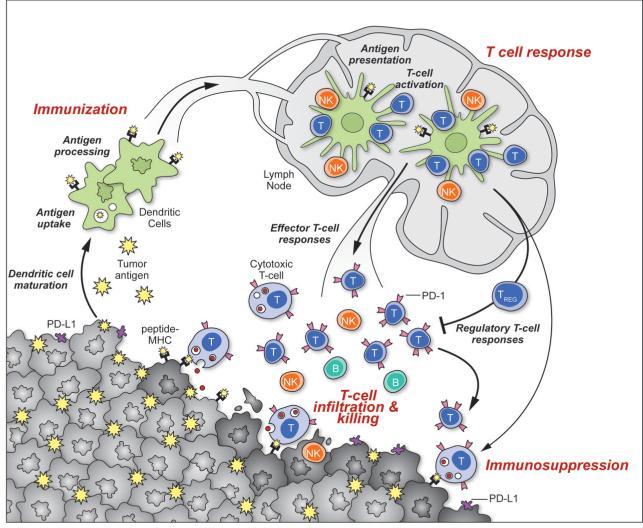
Figure 1. Generation and regulation of anti-tumor immunity.
Understanding the events in generating and regulating anti-tumor immunity suggests at least three sites for therapeutic intervention: promoting the antigen presentation functions of DCs, promoting the production of protective T cell responses, and overcoming immunosuppression in the tumor bed. Anti-tumor immune responses must begin with the capture of tumor-associated antigens by DCs, either delivered exogenously or captured from dead or dying tumor cells. The DCs process the captured antigen for presentation or cross-presentation on MHC class II and class I molecules (respectively) and migrate to draining lymph nodes. If capture and presentation occurred in the presence of an immunogenic maturation stimulus, DCs will elicit anti-cancer effector T cell responses in the lymph node; if no such stimulus was received, DCs will instead induce tolerance leading to T cell deletion, anergy, or the production of Tregs. In the lymph node, antigen presentation to T cells will elicit responses depending on the type of DC maturation stimulus received and on the interaction of T-cell costimulatory molecules with their surface receptors on DCs. Thus, interaction of CD28 or OX40 with CD80/86 or OX40L will promote potentially protective T cell responses, while interaction of CTLA4 with CD80/86 or PD-1 with PD-L1/PD-L2 will suppress T cell responses, and possibly promote Treg formation. Antigen-educated T cells (along with B cells and NK cells) will exit the lymph node and enter the tumor bed, where a host of immunosuppressive defense mechanisms can be produced by tumors (or infiltrating myeloid cells) that oppose effector T cell function. These include the upregulation of PD-L1/L2 on the cancer cell surface, release of PGE2, arginase and IDO (all T cell suppressors), and the release of VEGF (triggered in part by intratumoral hypoxia), which inhibits T cell diapedesis from the vasculature and thus infiltration into the tumor bed.