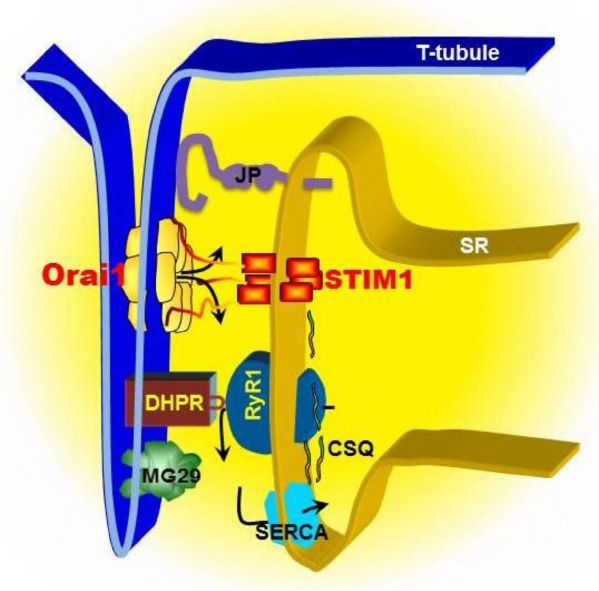
Fig. 1. Model of SOCE machinery in triad junction of skeletal muscle. SOCE in skeletal muscle displays rapid kinetics compared to non-excitable cells, which is likely attributed to a unique architecture between the plasma membrane (PM) and the sarcoplasmic reticulum (SR). In skeletal muscle, T-tubule invagination of the PM touches SR membranes. The close PM-SR junctional structure, known as “triad”, enables direct control of the RyR1/Ca2+ release channel by the DHPR via voltage-induced Ca2+ release. The released Ca2+ can be rapidly reuptake into SR by SERCA pump to complete the rapid contraction and relaxation cycle in skeletal muscle. STIM1s, as SR luminal Ca2+ binding proteins, can detect the reduction of Ca2+ concentration inside the SR lumen, undergo conformational changes, which in turn serves as a retrograde signal to activate Orai1 channel at T-tubule via direct contact interaction. Junctophilins (JP) and mitsugumin29 (MG29) are essential protein components of the junctional membrane structure between PM and SR. Disruption of JP or MG29 expression results in uncoupling of PM with SR, leading to dysfunction of SOCE. Alternatively, JP or MG29 may directly interact with RyR or other molecules involving in SOCE. Calsequestrin (CSQ) is a SR luminal Ca2+ binding protein, which influences the activation of SOCE by either altering the function of RyR or altering the Ca2+ homeostasis inside the SR lumen.