Abstract
Despite significant advances in the management of head trauma, there remains a lack of pharmacological treatment options for traumatic brain injury (TBI). While progesterone clinical trials have shown promise, corticosteroid trials have failed. The purpose of this study was to (1) characterize endogenous cerebrospinal fluid (CSF) progesterone and cortisol levels after TBI, (2) determine relationships between CSF and serum profiles, and (3) assess the utility of these hormones as predictors of long-term outcomes. We evaluated 130 adults with severe TBI. Serum samples (n=538) and CSF samples (n=746) were collected for 6 days post-injury, analyzed for cortisol and progesterone, and compared with healthy controls (n=13). Hormone data were linked with clinical data, including Glasgow Outcome Scale (GOS) scores at 6 and 12 months. Group based trajectory (TRAJ) analysis was used to develop temporal hormone profiles delineating distinct subpopulations. Compared with controls, CSF cortisol levels were significantly and persistently elevated during the first week after TBI, and high CSF cortisol levels were associated with poor outcome. As a precursor to cortisol, progesterone mediated these effects. Serum and CSF levels for both cortisol and progesterone were strongly correlated after TBI relative to controls, possibly because of blood–brain barrier disruption. Also, differentially impaired hormone transport and metabolism mechanisms after TBI, potential de novo synthesis of steroids within the brain, and the complex interplay of cortisol and pro-inflammatory cytokines may explain these acute hormone profiles and, when taken together, may help shed light on why corticosteroid trials have previously failed and why progesterone treatment after TBI may be beneficial.
Key words: : cortisol, group based trajectory analysis, outcome, progesterone, traumatic brain injury
Introduction
Despite significant advances in the management of head trauma and new developments in basic and clinical research, there remains a lack of pharmacological treatment options conferring significant neuroprotection after traumatic brain injury (TBI). Multiple studies, however, have evaluated the potential role that steroid hormones may have in reducing the adverse effects of secondary injury and improving outcome.
Multiple animal model studies report that acute progesterone therapy has pleiotropic effects on multiple components of secondary injury.1–5 Although progesterone's mechanisms of action are still being investigated, a growing body of literature have described its role as a potent neuroprotectant that reduces cerebral edema, modulates excitotoxicity, reconstitutes the blood–brain barrier (BBB), prevents neuronal loss, and improves functional outcomes.6–9 Similarly, allopregnanolone, a major progesterone metabolite, has been effective in mitigating the effects of secondary injury through multiple pathways, leading some to believe that progesterone's beneficial effects are from its metabolites rather than the molecule itself.10–12
To date, multiple clinical intervention studies evaluating the impact of progesterone treatment have shown promise for it as a neuroprotective agent.9,13 The Progesterone for the Treatment of Traumatic Brain Injury trial is now in phase III, randomized, double-blind, placebo-controlled testing based on earlier evidence of the potential benefit of administering progesterone acutely after moderate brain injury.9 A large clinical trial conducted in China has shown similar results and further revealed that patients receiving progesterone treatment experience better outcomes for up to 6 months after injury.13
Pre-clinical and clinical research has supported the use of methylprednisolone as an acute neuroprotectant after acute spinal cord injury (SCI).14–16 Because inflammatory changes contribute to neuronal damage, corticosteroids were thought to be beneficial after TBI. Yet, results have been mixed. In the 1970s, following reports of beneficial effects, the clinical use of corticosteroids after brain injury became fairly widespread.17,18 The literature suggested that corticosteroids reduced intracranial pressure and, with it, mortality.19,20 Although somewhat controversial, steroid supplementation has been thought to augment peripheral system hemodynamics and support some patients with acute adrenal insufficiency in the face of critical illness.21
Interestingly, additional clinical trials testing the protective effects of corticosteroids after TBI did not demonstrate a therapeutic effect. The Corticosteroid Randomization after Significant Head Injury trial was a randomized, placebo-controlled multicenter trial designed to determine the effect of high-dose methylprednisolone in 10,008 patients with TBI across 239 hospitals in 49 countries.22 This trial, however, was prematurely terminated because of unexpectedly higher mortality rates associated with the treatment arm, results that led to the elimination of steroid use from current treatment recommendations.
Progesterone is a hormone synthesized by the ovaries, placenta, and adrenal glands, and like all steroid hormones, is synthesized from cholesterol. Although classically considered a reproductive hormone in women, progesterone is now classified as a neurosteroid, having a variety of important roles in the CNS.23,24 Progesterone is also an indirect precursor of cortisol, a corticosteroid produced primarily in the adrenal cortex in response to the secretion of corticotropin-releasing hormone by the hypothalamus and subsequent release of adrenocorticotropic hormone by the anterior pituitary. Cortisol is widely recognized for its role in the stress response and for its physiologic anti-inflammatory effects. Excess cortisol, however, can have adverse effects on mood, cognition, and neurodegeneration.25–28 Interestingly, recent evidence supports the presence of synthetic enzymes needed for steroid synthesis in several human brain regions, including the hippocampus and amygdala.29–31 Although the exact function of these enzymes is still being investigated, work reported to date indicates the possibility of de novo synthesis of both progesterone and cortisol within the human brain.32
Despite what is known about hormones as neuroprotectants in the preclinical literature and the interest they have garnered in evaluating them as neuroprotectants in clinical populations, relatively little is known about early endogenous steroid hormone profiles in the periphery and their relationship to the central nervous system (CNS). Previously, we have reported that acute hypogonadotropic hypogonadism occurs among all patients with severe TBI and that adrenal hormone production, in addition to peripheral aromatization, are the primary contributors to residual serum hormone levels (e.g., estradiol, testosterone, progesterone, cortisol) during the first week post-injury.33 Interestingly, estradiol and testosterone levels in the periphery were not well correlated with CSF levels.34
Acute CSF progesterone and cortisol profiles have not been reported in the context of TBI, and their ability to discriminate outcomes has not been evaluated. Thus, the purpose of this study was to evaluate CSF progesterone and CSF cortisol profiles over the first week after severe TBI, determine relationships between these CSF profiles and concurrent serum profiles, and evaluate the sensitivity of these profiles in discriminating outcomes. We hypothesized that CSF cortisol levels would be elevated after TBI and be associated with adverse outcomes. As the synthetic precursor needed for cortisol synthesis, yet mechanistically neuroprotective, we hypothesized that CSF progesterone levels would also correlate with CSF cortisol levels, but carry less direct discriminatory capacity in predicting long-term outcome.
Methods
Study design and subjects
This study was approved by the Institutional Review Board at the University of Pittsburgh. We evaluated 130 adults with severe TBI at our level 1 trauma center. Subjects were enrolled if they were between the ages of 16 and 75, had a severe TBI based on an admission Glasgow Coma Score (GCS) ≤8 with positive findings on head computed tomography (CT), required an extraventricular drainage (EVD) catheter for intracranial pressure (ICP) monitoring and management, and had at least two CSF samples available for analysis. A subset of 111 patients also had serum samples available for analysis. Patients with penetrating head injury or with prolonged cardiac or respiratory arrest at injury were excluded from the study. Patients were also excluded if they had a history of pituitary or hypothalamic tumor, breast cancer necessitating chemotherapy treatment/tamoxifen, prostate cancer necessitating orchiectomy or luteinizing hormone suppression agents, or untreated thyroid disease.
While accurate information regarding hormone replacement therapy and oral contraception therapy was not obtainable for most women enrolled, women with TBI were not receiving hormone replacement or oral contraceptive therapy during the sample collection period. Further, subjects included in this cohort had to have Glasgow Outcome Scale (GOS) data at 6 months post-injury. For a variety of reasons (including loss to follow-up or refusal to complete tests), however, not all surviving subjects in the primary cohort (N=130) were able to complete 12-month outcomes. Therefore, a subset of subjects had a functional outcome measure at 12 months (n=111).
TBI subjects were admitted to the neurotrauma intensive care unit to receive treatment consistent with The Guidelines for the Management of Severe Head Injury.35 This included initial placement of an EVD, central venous catheter, and arterial catheter. When clinically necessary, surgical intervention for decompression of mass lesions was provided. Elevated ICP was treated in a stepwise fashion to regain control and maintain the pressure within normal parameters (<20 mm Hg), and cerebral perfusion pressure (CPP) was maintained at >60 mmHg. If CPP remained low, then mean arterial pressure (MAP) was supported with pressors or inotropes to keep MAP >90 mm Hg. Temperature was monitored, and a small subset of subjects received moderate hypothermia (temperature 32.5–33.5°C for 48 hours) if they were enrolled in a randomized controlled clinical trial evaluating hypothermia after severe TBI. All subjects not receiving hypothermia were treated to maintain a normothermic state. In total, 12 patients received hypothermia, and 118 remained normothermic.
Thirteen subjects were also involved in the Citicoline Brain Injury Treatment study, a randomized, double-blind, placebo-controlled, multi-center trial studying 90 days of citicoline treatment on functional outcome after TBI.36 Five subjects were randomly assigned to receive citicoline (1000 mg twice daily) and eight subjects were assigned to receive placebo beginning 24 h after injury.
As a comparison group, 13 healthy adult control subjects were separately enrolled, and samples were collected for CSF and serum hormone measurement. Control subjects were between 18 and 70 years old and had no current or past history of brain injury, neurological disease, or bleeding disorder. Women were excluded if pregnant, taking oral contraceptives or hormone replacement therapy at the time of injury, or had any history of reproductive or endocrine disorder. Control subjects' CSF was obtained via lumbar puncture for research purposes, and the procedure was not performed as a part of another clinical workup. Serum samples were collected during the same study visit. In addition, control women were interviewed about their reproductive history and menopausal status. Pre-menopausal women were sampled either in the follicular phase (days 5–10) or the luteal phase (days 18–23) of their cycle. For this study, there were six men and seven women in the healthy control population. Two women were in the follicular phase, four were in the luteal phase, and one woman was post-menopausal. Men and women were grouped together for cortisol analysis. Only men, women in the follicular phase, and post-menopausal women, however, were included when comparing progesterone levels to the TBI group.
Serum sample collection and measurements
Blood samples (n=538) were collected at approximately 7:00 AM daily for the first 6 days after injury. Fifty-nine subjects had at least one sampling day when the blood sample was collected in the evening (7:00 PM) in addition to, or instead of, the morning sample. On collection, each sample was centrifuged, aliquoted in polypropylene cryovials, and the serum was stored at −80°C until the time of assay. For control subjects, blood was drawn at approximately 7:00 AM, processed, and stored for later batch analysis.
Serum cortisol and progesterone, in addition to CSF cortisol, were measured using a radioimmunoassay with the Coat-A-Count® In-vitro Diagnostic Test Kit (Siemens Healthcare Diagnostics Inc., Los Angeles, CA). Each kit was a solid-phase 125I radioimmunoassay designed for the direct, quantitative measurement of each hormone in serum using 25 μL (cortisol) or 100 μL (progesterone) sample aliquots. The interassay and intra-assay coefficients of variation (CV) were less than 10% for these assays. Samples with out of range (low) levels were assigned the detection limit of the respective assay. Samples with levels that were undetectable were assigned values of 0.001 for analysis purposes.
CSF sample collection and measurements
CSF samples (n=746) were collected passively via EVD. CSF was collected up to 2 times daily (morning and evening) for up to 6 days after injury. After collection, the sample was stored at 4°C until processing. CSF samples were then centrifuged, aliquoted, and stored at −80°C until batch analysis. For control subjects, CSF was collected at approximately 7:00 AM via a single lumbar puncture, processed, and stored for later batch analysis.
Enzyme-linked immunosorbent assay (ELISA), (Salimetrics) was used to measure CSF progesterone. The inter-ELISA and intra-ELISA CVs were less than 10%. CSF samples were run in duplicate, with the average value for each sample used in analysis. Samples with out of range (low) levels were assigned the detection limit of the respective assay. Samples with levels that were undetectable were assigned values of 0.001.
Demographic and clinical injury variables
Independent variables included sex, age, body mass index (BMI), GCS, injury severity score (ISS), hospital length of stay (LOS), the number of acute care complications, mechanism of injury, radiological injury type, hypothermia treatment, and citicoline treatment. The best GCS score taken within 24 h of injury was recorded. Injury type was abstracted from clinical head CT radiology reports.
Outcome variables
Mortality during acute care hospitalization was determined by medical record review and/or the Social Security Death Index (http://ssdi.rootsweb.ancestry.com/). GOS, Disability Rating Scale (DRS) and the cognitive component of the functional independence measure (FIM) (FIM-Cog) served as the primary outcome measures. GOS is a frequently used measure developed by Jennett and Bond.37 GOS scores were assigned to TBI subjects at 6 months and 12 months after injury. The five categories of the scale are: dead (1), vegetative (2), severely disabled (3), moderately disabled (4), and good recovery (5). For the purposes of this study, GOS categories were collapsed into 1 vs. 2/3 vs. 4/5.
The DRS, developed by Rappaport and associates,38 is a measure that rates persons on four categories: arousal and awareness, cognitive ability to complete self-care functions, physical dependence on others, and psychosocial adaptability for work, housework, and school. At 6 and 12 months post-injury, patients were assigned a DRS score ranging from no disability (0) to dead (30). For this study, subjects were separated into three groups based on scores: 0–3 with partial to no disability (1), 4–14 with moderate or severe disability (2) and 15–30 with extremely severe disability, vegetative state, or dead (3).
The FIM is a well-established measure of functional independence.39 The measure is routinely used during acute rehabilitation as a reliable indicator of patient progress and is also used to assess long-term outcome after injury.40,41 This measure consists of 18 items composed of 13 motor tasks and 5 cognitive tasks. Each task is rated on a 7-point ordinal scale ranging from complete dependence to complete independence. For this study, the 5 cognitive measures (FIM-Cog) were analyzed independently from the 13 motor measures (FIM-motor), and the sum of a subject's scores for the cognitive subscales was used for multivariate analysis. In addition, based on their FIM scores, subjects were dichotomized into two groups for bivariate analysis: (1) independent/supervision and 2) mildly to totally dependent. Subjects receiving scores ≥5 on every measure were assigned to the independent/supervision group. A subject scoring <5 on any task was assigned to the mildly to totally dependent group. This grouping is based on a previously documented four level categorization of the FIM instrument.42
Statistical analysis
Before analysis, multiple hormone values from the same day for the same subject were averaged, leaving a total of 337 serum hormone values and 504 CSF hormone values from the first 6 days post-injury. At this point, nine outlier hormone values greater than 5 standard deviation outside of the mean were removed from the data set before analysis. Each subject's mean hormone value across the 6-day sampling period was then calculated. In addition, the daily mean hormone values for all subjects with TBI were calculated and compared with control levels. Statistical analyses were performed using SPSS Version 20.0 (Chicago, IL) and SAS Version 9.2 (Cary, NC).
Descriptive statistics, including mean, standard error of the mean, and median were computed for all continuous variables. Frequencies and percentages were determined for categorical variables. Normality was assessed for all continuous variables using the Kolmogorov-Smirnov test. An independent samples t test was used when no violations of the normality assumption were observed. Otherwise, a Mann Whitney test or Kruskal-Wallis test was used, as appropriate. Chi-square, with the Fisher exact test when appropriate, was used to determine associations between categorical variables. Associations between continuous variables were assessed using Pearson correlations. All tests were two tailed, with significance set at α=0.05.
Group based trajectory analysis (TRAJ) was used to develop temporal hormone profiles that delineate distinct subpopulations in the cohort similar to that previously described.33,43,44 TRAJ is a specialized application of finite mixture modeling that assesses patterns of change over time,45 and models were estimated using the PROC TRAJ Macro46 for SAS software. The TRAJ procedure uses a probability function to discern a set of trajectories that closely resemble one another. TRAJ assumes the existence of unobserved (latent) subpopulations, and leverages the power of repeated sampling to relate temporal patterns. Using this approach, TRAJ analyses can identify clusters of persons with similar CSF hormone profiles across time. For multivariate analyses described below, some TRAJ groups were combined for analysis.
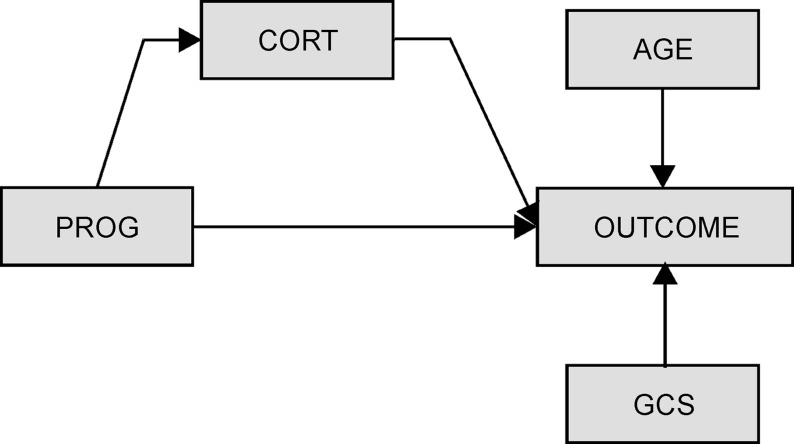
We examined bivariate associations between demographic/clinical variables and outcome variables to identify variables that should be controlled for in the multivariate models. Multivariate models were used to evaluate how biomarker trajectory group membership affected GOS, DRS, and FIM-Cog scores at 6 and 12 months post-TBI. Because progesterone is a synthetic precursor to other hormones like cortisol, structural equations modeling (SEQM) was used to characterize CSF progesterone and cortisol profiles simultaneously in relationship to each other and to outcome, while adjusting for other relevant variables observed to be significantly related to outcome in bivariate analysis (Fig. 1).
FIG. 1.
Theoretical structural equations modeling figure depicting cerebrospinal fluid cortisol (cort) relationships with outcome and direct and indirect effects of progesterone (prog) on outcome via its relationship with cortisol. GCS, Glasgow Coma Score.
We have used this approach previously to examine steroid hormone pathways in the context of acute TBI.33 Clinical and demographic variables having a p value≤0.2 when compared with outcomes in bivariate analyses were assessed in multivariate SEQM models. Within each model, both direct and indirect (mediating) effects were assessed for progesterone. A p value≤0.05 was considered statistically significant. Mean hormone values for the entire time course were explored in multivariate analysis (data not shown). Similar to other CSF biomarkers, however,44 TRAJ based multivariate models were superior in predicting outcome, and thus are reported in the results.
Results
Description of the cohort
The mean age of the cohort was 35.57±1.36 years, with an age range of 16–73 years and women representing 21% of the population. The primary mechanism of injury among both men and women was motor vehicle collisions (46%) followed by motorcycle collisions (21%). The median GCS score for the cohort was 6.5, and the mean ISS score was 33.98±0.85. The most common injury types observed on CT scan were subarachnoid hemorrhage (74%) and subdural hematoma (62%). This population had an average of 2.23±0.16 complications and a 23% acute care mortality rate. The mean hospital LOS was 21.15±0.961 days. The average BMI was 26.49±0.476.
Men in the cohort were, on average, significantly younger than women (34.15±1.50 yrs vs. 41.00±2.96 years; p<0.05). Men also had more subdural hematomas than women (68.0% vs. 40.7%; p<0.05). There were not any sex differences, however, among other radiographic injury types observed on acute care CT scan. Motorcycle collisions were more likely to be the cause of injury among men (25.2% vs. 3.7%; p<0.05). There were no sex differences observed for any of the other variables, including interventional study participation, hormone levels, and outcome measures at 6 and 12 months. At 6 months post-injury, 24.6% of the population had a GOS score of 1, 40% had a GOS of 2 or 3, and 35.4% had a GOS of 4 or 5. Control hormone values are outlined in Table 1. In our control population (n=13), 53.8% were women, and the average age was 34.88±14.53 years.
Table 1.
Control Population Description
| Mean cortisol levels±SE | ||
|---|---|---|
| CSF (ng/mL) | Serum (ng/mL) | |
| Men (n=6) | 4.30±0.83 | 137.97±23.65 |
| Women (n=7) | 4.67±0.36 | 130.73±14.45 |
| All (n=13) | *4.50±0.41 | *134.07±12.84 |
| Mean progesterone levels±SE | ||
|---|---|---|
| CSF (pg/mL) | Serum (ng/mL) | |
| Men (n=6) | 28.85±14.06 | 0.82±0.12 |
| Post-menopausal women (n=1) | 21.40 | 0.574 |
| Pre-menopausal women (n=6) | 108.88±39.31 | 9.50±4.02 |
| Follicular phase (n=2) | 16.95±8.75 | 0.76±0.16 |
| Luteal phase (n=4) | 154.85±41.69 | 13.87±4.61 |
| Men+women not in luteal phase (n=9) | *25.38±9.36 | *0.78±0.09 |
Denotes control means used for analysis.
SE, standard error; CSF, cerebrospinal fluid.
Mean hormone levels by day
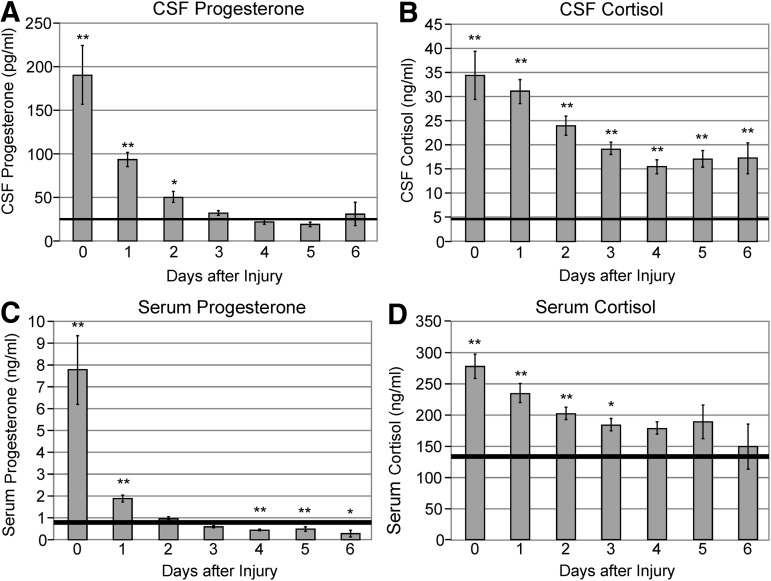
Figures 2A,B illustrate mean CSF progesterone and cortisol levels by day compared with healthy controls. Mean CSF progesterone levels were significantly higher than controls on day 0–1 (p<0.01) and day 2 (p<0.05), and then remained at control levels for the remainder of the sampling period. Mean CSF cortisol levels were higher than control values on all days sampled (p<0.01 all comparisons).
FIG. 2.
(A) Daily mean cerebrospinal fluid (CSF) progesterone levels. Mean CSF progesterone levels were elevated compared with controls on days 0–1 (p<0.01) and day 2 (p<0.05). (Solid line=control mean for men plus women in menopause or follicular phase of their menstrual cycle.) (B) Daily mean CSF cortisol levels. Mean CSF cortisol levels were significantly elevated for subjects with traumatic brain injury compared with controls on all days sampled (p<0.01). (Solid line=control mean for men plus women.) (C) Daily mean serum progesterone levels. Mean serum progesterone levels were significantly elevated compared with controls on days 0–1 (p<0.01) and then fell significantly below controls on days 4–5 (p<0.01) and day 6 (p<0.05). (Solid line=control mean for men plus women in menopause or follicular phase of their menstrual cycle.) (D) Daily mean serum cortisol levels. Mean serum cortisol levels were elevated compared with controls on days 0–2 (p<0.01) and day 3 (p<0.05). (Solid line=control mean for men plus women.)
Figures 2C,D illustrate mean serum progesterone and cortisol levels by day compared with healthy controls. Serum progesterone levels were significantly elevated compared with controls on day 0–1 (p<0.01), then fell significantly below controls on days 4–5 (p<0.01) and day 6 (p<0.05). Mean serum cortisol levels were elevated compared with controls on days 0–2 (p<0.01) and day 3 (p<0.05), and then remained at or near control levels on days 4–6.
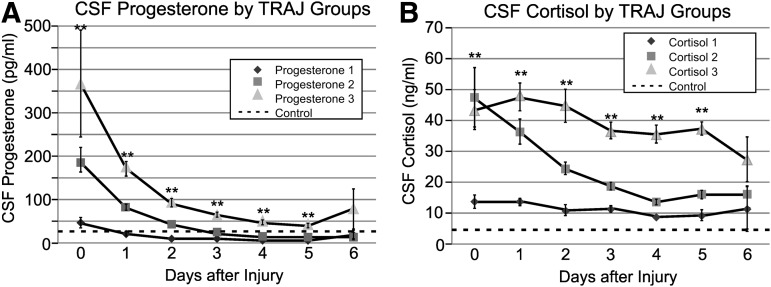
CSF hormone trajectory profiles
There were three distinct TRAJ group profiles identified for CSF progesterone (Fig. 3A): high, middle, and low groups. Progesterone levels variably declined over time for each TRAJ group, and levels for each group were significantly different from each other on days 0–5 post-TBI (p<0.01 all comparisons). Progesterone levels for the high group were significantly higher than control levels on days 0–5 post-TBI (p<0.01, all comparisons). Progesterone levels for the middle group were significantly higher than control levels only on days 0–2 (p<0.01, all comparisons). Progesterone levels for the low group were comparable to controls on days 0–1, but lower than controls on days 2–3 (p<0.05, all comparisons) and days 4–5 (p<0.01, all comparisons).
FIG. 3.
(A) Daily mean cerebrospinal fluid (CSF) progesterone levels for progesterone trajectory (TRAJ) groups compared with healthy controls. TRAJ group progesterone levels were significantly different on days 0–5 (p<0.01). Progesterone levels for the high group were significantly higher than control levels on days 0–5 post-TBI (p<0.01, all comparisons). Progesterone levels for the middle group were significantly higher than control levels only on days 0–2 (p<0.01, all comparisons). Progesterone levels for the low group were comparable to controls on days 0–1, but lower than controls on days 2–3 (p<0.05, all comparisons) and days 4–5 ( p<0.01, all comparisons). (Dotted line=control mean for men plus women in menopause or follicular phase of their menstrual cycle.) (B) Daily mean CSF cortisol levels for cortisol TRAJ groups compared with healthy controls. TRAJ group cortisol levels were significantly different on days 0–5 (p<0.01). Cortisol levels for the high and middle groups were significantly higher than control levels on days 0–6 (p<0.01, all comparisons), and cortisol levels for the low group were higher than controls on days 0–5 (p<0.01, all comparisons). (Dotted line=control mean for men plus women.)
There were also three distinct TRAJ group profiles identified for CSF cortisol (Fig. 3B): high, middle, and low groups. The high and middle groups had comparably elevated cortisol levels on day 0, but levels for the high group remained consistently higher than the other two groups throughout the remaining sampling period. Cortisol levels for each TRAJ were significantly different from each other on days 0–5 post-TBI (p<0.01). Cortisol levels for the high and middle groups were significantly higher than control levels on days 0–6 (p<0.01, all comparisons), and cortisol levels for the low group were higher than controls on days 0–5 (p<0.01, all comparisons).
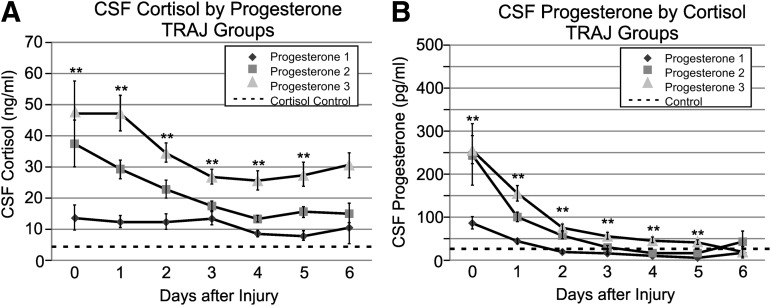
We graphed daily cortisol levels by progesterone TRAJ group to evaluate the utility of using progesterone TRAJ to distinguish differences in CSF cortisol levels. Figure 4A shows that CSF cortisol levels were significantly different between groups on days 0–5 (p<0.01 all comparisons). CSF cortisol levels by progesterone TRAJ group over time (Fig. 4A) resemble CSF cortisol levels by cortisol TRAJ group over time (Fig. 3B), suggesting a dependence of cortisol levels on progesterone substrate. The elevated cortisol levels over time suggest that there is enough progesterone substrate to support ongoing cortisol synthesis. Figure 4B shows that daily mean CSF progesterone levels for each cortisol TRAJ group rapidly decline over time. Progesterone levels were significantly different between groups on days 0–5 (p<0.01 all comparisons).
FIG. 4.
(A) Daily mean cerebrospinal fluid (CSF) cortisol levels for progesterone trajectory (TRAJ) groups compared with healthy controls. Progesterone TRAJ group cortisol levels were significantly different on days 0–5 (p<0.01). (Dotted line=control mean for men plus women.) (B) Daily mean CSF progesterone levels for cortisol trajectory groups compared with healthy controls. Cortisol TRAJ group progesterone levels were significantly different on days 0–5 (p<0.01). (Dotted line=control mean for men plus women in menopause or follicular phase of their menstrual cycle.)
Bivariate analyses: TRAJ associations with outcome
Table 2 summarizes cortisol and progesterone TRAJ associations with outcome at 6 and 12 months. Subjects grouped in the middle and high cortisol TRAJ groups (groups 2 and 3) were more likely to have worse GOS scores and worse DRS scores at 6 and 12 months (p<0.01 all comparisons). Subjects in the middle and high cortisol TRAJ groups also had lower scores on the FIM-Cog at 6 and 12 months (p<0.01 all comparisons). Although bivariate associations between progesterone TRAJ group and outcome were not as strong, subjects grouped together in the middle and high progesterone groups were more likely to have worse GOS scores at 6 months (p<0.05) and lower scores on the FIM-Cog measure at 6 and 12 months (p<0.05 all comparisons).
Table 2.
Bivariate Analysis—Cerebrospinal Fluid Hormone Trajectory Group by Outcome
| 6 month outcomes | 12 month outcomes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cortisol TRAJ 1 | Cortisol TRAJ 2–3 | p value | Progesterone TRAJ 1 | Progesterone TRAJ 2–3 | p value | Cortisol TRAJ 1 | Cortisol TRAJ 2–3 | p value | Progesterone TRAJ 1 | Progesterone TRAJ 2–3 | p value | |
| GOS | ||||||||||||
| # GOS 1 (%) | 6 (14.0) | 26 (29.9) | 0.008 | 4 (14.8) | 28 (27.2) | 0.040 | 6 (15.8) | 26 (35.6) | 0.002 | 4 (18.2) | 28 (31.5) | 0.175 |
| # GOS 2/3 (%) | 14 (32.6) | 38 (43.7) | 9 (33.3) | 43 (41.7) | 6 (15.8) | 23 (31.5) | 4 (18.2) | 25 (28.1) | ||||
| # GOS 4/5 (%) | 23 (53.5) | 23 (26.4) | 14 (51.9) | 32 (31.1) | 26 (68.4) | 24 (32.9) | 14 (63.6) | 36 (40.4) | ||||
| DRS | ||||||||||||
| # DRS 1 (%) | 21 (50.0) | 19 (21.8) | 0.002 | 13 (50.0) | 27 (26.2) | 0.081 | 21 (56.8) | 19 (27.1) | 0.004 | 11 (52.4) | 29 (33.7) | 0.352 |
| # DRS 2 (%) | 12 (28.6) | 27 (31.0) | 6 (23.1) | 33 (32.0) | 9 (24.3) | 17 (24.3) | 4 (19.0) | 22 (25.6) | ||||
| # DRS 3 (%) | 9 (21.4) | 41 (47.1) | 7 (26.9) | 43 (41.7) | 7 (18.9) | 34 (48.6) | 6 (28.6) | 35 (40.7) | ||||
| Mean FIM-Cog±SE | 30.15±1.39 | 23.13±1.47 | <0.001 | 29.50±2.19 | 24.74±1.28 | 0.022 | 31.22±1.07 | 26.46±1.49 | 0.002 | 30.25±2.51 | 27.79±1.15 | 0.030 |
TRAJ, trajectory; GOS, Glasgow Outcome Scale; DRS, Disability Rating Scale; FIM-Cog, functional independence measure-cognitive; SE, standard error.
Mean serum cortisol and progesterone comparisons with outcome were not significant for any measure (Table 3A,B).
Table 3A.
Descriptive Comparisons of Covariates by 6-Month Outcome
| GOS | DRS | FIM-Cog | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dead (GOS=1) | Veg–severe (GOS=2/3) | Mod–good (GOS=4/5) | p value | Minimal (DRS=0–3) | Mod–severe (DRS=4–14) | Veg–dead (DRS=15–3) | p value | Independent | Dependent | p value | |
| GCS, median | 6 | 6 | 7 | 0.062 | 7 | 7 | 6 | 0.043 | 7 | 6 | 0.231 |
| BMI, mean±SE | 26.66±0.98 | 25.77±0.75 | 27.20±0.80 | 0.314 | 26.13±0.90 | 26.95±0.86 | 26.52±0.76 | 0.627 | 27.16±0.98 | 25.85±0.61 | 0.988 |
| Age, mean±SE | 46.09±2.94 | 34.27±2.01 | 29.72±1.77 | 0.000 | 27.93±1.84 | 35.95±2.28 | 41.26±2.38 | 0.001 | 30.07±1.72 | 32.56±2.68 | 0.637 |
| ISS, mean±SE | 34.38±1.80 | 35.00±1.32 | 32.54±1.41 | 0.538 | 32.45±1.52 | 33.49±1.55 | 35.74±1.38 | 0.308 | 32.24±1.39 | 35.04±1.80 | 0.230 |
| LOS, mean±SE | 13.94±1.84 | 26.21±1.51 | 20.46±1.21 | 0.000 | 20.38±1.34 | 24.67±1.71 | 19.32±1.74 | 0.111 | 19.73±1.10 | 29.11±2.11 | 0.000 |
| Mechanism of injury (#, %) | |||||||||||
| Motor vehicle | 8 (25.0) | 25 (48.1) | 27 (58.7) | 0.014 | 24 (60.0) | 17 (43.6) | 18 (36.0) | 0.097 | 24 (53.3) | 12 (44.4) | 0.578 |
| Motorcycle | 6 (18.8) | 13 (25.0) | 8 (17.4) | 6 (15.0) | 12 (30.8) | 9 (18.0) | 9 (20.0) | 9 (33.3) | |||
| Fall | 12 (37.5) | 5 (9.6) | 4 (8.7) | 3 (7.5) | 5 (12.8) | 13 (26.0) | 4 (8.9) | 3 (11.1) | |||
| Other | 6 (18.8) | 9 (17.3) | 7 (15.2) | 7 (17.5) | 5 (12.8) | 10 (20.0) | 8 (17.8) | 3 (11.1) | |||
| Radiological injury type (#, %) | |||||||||||
| SDH | 23 (71.9) | 36 (69.2) | 22 (47.8) | 0.046 | 21 (52.5) | 24 (61.5) | 36 (72.0) | 0.167 | 26 (57.8) | 17 (63.0) | 0.805 |
| DAI | 6 (18.8) | 15 (28.8) | 20 (43.5) | 0.061 | 20 (50.0) | 8 (20.5) | 12 (24.0) | 0.009 | 17 (37.8) | 7 (25.9) | 0.439 |
| EDH | 5 (15.6) | 4 (7.7) | 11 (23.9) | 0.084 | 10 (25.0) | 4 (10.3) | 6 (12.0) | 0.163 | 7 (15.6) | 4 (14.8) | 1.000 |
| SAH | 28 (87.5) | 38 (73.1) | 30 (65.2) | 0.079 | 26 (65.0) | 27 (69.2) | 42 (84.0) | 0.088 | 31 (68.9) | 22 (81.5) | 0.281 |
| Contusion | 21 (65.6) | 22 (42.3) | 18 (39.1) | 0.049 | 16 (40.0) | 16 (41.0) | 28 (56.0) | 0.237 | 20 (44.4) | 11 (40.7) | 0.810 |
| IVH | 8 (25.0) | 15 (28.8) | 14 (30.4) | 0.883 | 12 (30.0) | 10 (25.6) | 15 (30.0) | 0.911 | 10 (22.2) | 8 (29.6) | 0.577 |
| ICH | 10 (31.2) | 20 (38.5) | 15 (32.6) | 0.818 | 10 (25.0) | 19 (48.7) | 16 (32.0) | 0.080 | 13 (28.9) | 12 (44.4) | 0.208 |
| COBRIT study (#, %) | |||||||||||
| Citicoline | 1 (33.3) | 1 (20.0) | 3 (60.0) | 0.767 | 2 (50.0) | 2 (40.0) | 1 (25.0) | 1.000 | 2 (33.3) | 2 (66.7) | 0.524 |
| Placebo | 2 (66.7) | 4 (80.0) | 2 (40.0) | 2 (50.0) | 3 (60.0) | 3 (75.0) | 4 (66.7) | 1 (33.3) | |||
| Hypothermia study (#, %) | |||||||||||
| Hypothermia | 0 (0) | 3 (50.0) | 9 (90.0) | 0.060 | 7 (87.5) | 4 (66.7) | 0 (0) | 0.093 | 9 (90.0) | 1 (100) | 1.000 |
| Control | 1 (100) | 3 (50.0) | 1 (10.0) | 1 (12.5) | 2 (33.3) | 2 (100) | 1 (10.0) | 0 (0) | |||
| Hormone levels (mean±SE) | |||||||||||
| CSF cortisol | 29.53±2.97 | 23.50±1.77 | 18.13±1.62 | 0.002 | 18.28±1.71 | 22.49±2.04 | 27.66±2.22 | 0.003 | 19.62±1.80 | 26.56±2.56 | 0.009 |
| Serum cortisol | 225.00±20.20 | 198.93±9.19 | 193.65±9.48 | 0.791 | 178.69±8.45 | 213.55±11.72 | 216.11±14.64 | 0.115 | 200.16±10.16 | 218.79±13.65 | 0.190 |
| CSF progesterone | 87.72±16.21 | 57.62±5.90 | 40.83±5.13 | 0.004 | 42.23±5.58 | 55.10±6.92 | 76.60±11.07 | 0.022 | 40.40±4.30 | 76.15±9.95 | 0.002 |
| Serum progesterone | 2.19±0.58 | 1.33±0.16 | 1.38±0.28 | 0.204 | 1.25±0.29 | 1.39±0.19 | 1.96±0.42 | 0.136 | 1.34±0.27 | 1.58±0.28 | 0.195 |
p values based on Fisher exact test, Mann Whitney or Kruskal-Wallis as appropriate.
GOS, Glasgow Outcome Scale; DRS, Disability Rating Scale; FIM-Cog, functional independence measure-cognitive; veg, vegetative; mod, moderate; GCS, Glasgow Coma Score; BMI, body mass index; SE, standard error; ISS, injury severity score; LOS, length of stay; SDH, subdural hematoma; DAI, diffuse axonal injury; EDH, epidural hematoma; SAH, subarachnoid hemorrhage; IVH, intraventricular hemorrhage; ICH, intracerebral hemorrhage; COBRIT, citicoline brain injury treatment; CSF, cerebrospinal fluid.
Table 3B.
Descriptive Comparisons of Covariates by 12-Month Outcome
| GOS | DRS | FIM-Cog | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dead (GOS=1) | Veg–severe (GOS=2/3) | Mod–good (GOS=4/5) | p value | Minimal (DRS=0-3) | Mod–severe (DRS=4-14) | Veg–dead (DRS=15-30) | p value | Independent | Dependent | p value | |
| GCS, median | 6 | 6 | 7 | 0.316 | 7 | 6 | 6 | 0.038 | 7 | 6 | 0.123 |
| BMI, mean±SE | 26.66±0.98 | 25.52±0.76 | 26.82±0.82 | 0.714 | 26.08±0.81 | 26.27±1.10 | 26.66±0.84 | 0.760 | 26.70±0.92 | 26.76±0.93 | 0.490 |
| Age, mean±SE | 46.09±2.94 | 34.41±2.62 | 30.12±1.92 | 0.000 | 30.98±2.27 | 31.73±2.70 | 43.44±2.60 | 0.001 | 30.86±1.79 | 32.71±3.67 | 0.887 |
| ISS, mean±SE | 34.38±1.80 | 38.62±1.86 | 32.08±1.22 | 0.063 | 32.50±1.35 | 36.65±2.26 | 34.83±1.54 | 0.619 | 33.16±1.49 | 32.82±2.18 | 0.766 |
| LOS, mean±SE | 13.94±1.84 | 28.03±2.09 | 19.54±1.00 | 0.000 | 18.23±0.95 | 27.42±2.16 | 17.17±1.90 | 0.001 | 19.58±1.05 | 27.82±2.63 | 0.008 |
| Mechanism of injury (#, %) | |||||||||||
| Motor vehicle | 8 (25.0) | 14 (48.3) | 29 (58.0) | 0.007 | 22 (55.0) | 14 (53.8) | 13 (31.7) | 0.042 | 25 (58.1) | 6 (35.3) | 0.360 |
| Motorcycle | 6 (18.8) | 6 (20.7) | 11 (22.0) | 9 (22.5) | 5 (19.2) | 7 (17.1) | 9 (20.9) | 5 (29.4) | |||
| Fall | 12 (37.5) | 2 (6.9) | 4 (8.0) | 4 (10.0) | 1 (3.8) | 13 (31.7) | 2 (4.7) | 2 (11.8) | |||
| Other | 6 (18.8) | 7 (24.1) | 6 (12.0) | 5 (12.5) | 6 (23.1) | 8 (19.5) | 7 (16.3) | 4 (23.5) | |||
| Radiological injury type (#, %) | |||||||||||
| SDH | 23 (71.9) | 19 (65.5) | 27 (54.0) | 0.270 | 24 (60.0) | 13 (50.0) | 31 (75.6) | 0.086 | 26 (60.5) | 9 (52.9) | 0.772 |
| DAI | 6 (18.8) | 7 (24.1) | 21 (42.0) | 0.064 | 17 (42.5) | 7 (26.9) | 8 (19.5) | 0.079 | 18 (41.9) | 22 (11.8) | 0.034 |
| EDH | 5 (15.6) | 4 (13.8) | 11 (22.0) | 0.697 | 10 (25.0) | 5 (19.2) | 5 (12.2) | 0.338 | 8 (18.6) | 2 (11.8) | 0.709 |
| SAH | 28 (87.5) | 22 (75.9) | 32 (64.0) | 0.057 | 27 (67.5) | 19 (73.1) | 33 (80.5) | 0.409 | 31 (72.1) | 11 (64.7) | 0.755 |
| Contusion | 21 (65.6) | 11 (37.9) | 22 (44.0) | 0.066 | 18 (45.0) | 8 (30.8) | 25 (61.0) | 0.054 | 17 (39.5) | 6 (35.3) | 1.000 |
| IVH | 8 (25.0) | 8 (27.6) | 13 (26.0) | 1.000 | 9 (22.5) | 8 (30.8) | 11 (26.8) | 0.755 | 8 (18.6) | 5 (29.4) | 0.488 |
| ICH | 10 (31.2) | 14 (48.3) | 15 (30.0) | 0.242 | 12 (30.0) | 12 (46.2) | 14 (34.1) | 0.424 | 14 (32.6) | 7 (41.2) | 0.560 |
| COBRIT study (#, %) | |||||||||||
| Citicoline | 1 (33.3) | 1 (20.0) | 3 (60.0) | 1.000 | 3 (75.0) | 1 (25.0) | 1 (33.3) | 0.455 | 3 (50.0) | 1 (50.0) | 1.000 |
| Placebo | 2 (66.7) | 2 (80.0) | 2 (40.0) | 1 (25.0) | 3 (75.0) | 2 (66.7) | 3 (50.0) | 1 (50.0) | |||
| Hypothermia study (#, %) | |||||||||||
| Hypothermia | 0 (0) | 0 (0) | 10 (83.3) | 0.231 | 7 (77.8) | 3 (100) | 0 (0) | 0.329 | 8 (88.9) | 1 (100) | 1.000 |
| Control | 1 (100) | 0 (0) | 2 (16.7) | 2 (22.2) | 0 (0) | 1 (100) | 1 (11.1) | 0 (0) | |||
| Hormone levels (mean±SE) | |||||||||||
| CSF cortisol | 29.53±2.97 | 24.38±1.98 | 17.76±1.67 | 0.000 | 19.60±2.12 | 20.96±2.16 | 27.22±2.43 | 0.020 | 20.65±1.97 | 22.06±2.40 | 0.314 |
| Serum cortisol | 225.00±20.20 | 211.14±11.29 | 192.37±10.10 | 0.426 | 195.18±11.26 | 206.55±14.28 | 219.24±17.08 | 0.737 | 210.24±10.56 | 201.58±18.79 | 0.726 |
| CSF progesterone | 87.72±16.21 | 68.83±9.64 | 38.40±4.09 | 0.002 | 46.23±6.74 | 58.77±9.08 | 77.12±13.10 | 0.077 | 49.30±6.03 | 63.71±12.47 | 0.545 |
| Serum progesterone | 2.19±0.58 | 1.29±0.24 | 1.33±0.25 | 0.162 | 1.09±0.19 | 1.59±0.30 | 1.92±0.50 | 0.281 | 1.16±0.20 | 1.67±0.41 | 0.267 |
p values based on Fisher exact test, Mann Whitney or Kruskal Wallis as appropriate.
GOS, Glasgow Outcome Scale; DRS, Disability Rating Scale; FIM-Cog, functional independence measure-cognitive; veg, vegetative; mod, moderate; GCS, Glasgow Coma Score; BMI, body mass index; SE, standard error; ISS, injury severity score; LOS, length of stay; SDH, subdural hematoma; DAI, diffuse axonal injury; EDH, epidural hematoma; SAH, subarachnoid hemorrhage; IVH, intraventricular hemorrhage; ICH, intracerebral hemorrhage; COBRIT, citicoline brain injury treatment; CSF, cerebrospinal fluid.
Bivariate analyses: Covariates by outcome
Age was significantly correlated with GOS and DRS at both 6 and 12 months, with younger subjects having a higher acute care survival rate and better outcome scores (p<0.01 all comparisons). Also, age was correlated with mean CSF cortisol (r=0.270, p<0.01), serum cortisol (r=0.216, p<0.05), and CSF progesterone (r=0.260, p<0.01). In addition, a shorter acute care hospital length of stay was associated with better outcome on all measures except for DRS at 6 months (p<0.01 all comparisons). A lower GCS was associated with worse DRS scores (p<0.05) at both 6 and 12 months. Additional injury severity, injury mechanism, and injury type covariate associations with 6 and 12 month outcomes are provided in Table 3A,B. Hypothermia treatment and citicoline treatment were not associated with any outcome in this cohort.
Multivariate analysis: Associations with outcome
Multivariate analysis suggests that, after adjusting for age and GCS, middle and high CSF cortisol TRAJ group membership tended to be associated with GOS scores at 6 months (p<0.09) and significantly associated with GOS scores at 12 months (p<0.05), where the TRAJ groups with higher CSF cortisol levels were associated with increased risk for worse outcome (Table 4). Middle and high CSF cortisol TRAJ group membership was also significantly associated with worse DRS scores at 6 and 12 months (p<0.05), as well as lower FIM-cog scores at 6 months (p<0.01) and 12 months (p<0.05). In each of these multivariate models, middle and high CSF progesterone TRAJ group membership was significantly and highly associated with middle and high CSF cortisol TRAJ group membership (p<0.001 all comparisons except p<0.05 for FIM-Cog at 12 months. While progesterone TRAJ group membership did not directly influence any outcome at either the 6 or 12 month time points, progesterone TRAJ group membership did have a significant/trending indirect effect on outcome for many of the measures at each of the time points via its effects on cortisol TRAJ group membership (Table 4).
Table 4.
Structural Equations Modeling Multivariate Analysis
| Variable | Standardized coefficients | p value |
|---|---|---|
| GOS at 6 months | ||
| Age | 0.39 | <0.001 |
| GCS | 0.24 | 0.002 |
| Progesterone | −0.03 | 0.733 |
| Cortisol | −0.15 | 0.088 |
| Progesterone on cortisol | 0.46 | <0.001 |
| Indirect effect | −0.07 | 0.102 |
| GOS at 12 months | ||
| Age | 0.40 | <0.001 |
| GCS | 0.19 | 0.029 |
| Progesterone | −0.01 | 0.949 |
| Cortisol | −0.20 | 0.031 |
| Progesterone on cortisol | 0.37 | <0.001 |
| Indirect effect | −0.07 | 0.056 |
| DRS at 6 months | ||
| Age | 0.34 | <0.001 |
| GCS | −0.27 | 0.001 |
| Progesterone | 0.04 | 0.622 |
| Cortisol | 0.21 | 0.017 |
| Progesterone on cortisol | 0.45 | <0.001 |
| Indirect effect | 0.09 | 0.027 |
| DRS at 12 months | ||
| Age | 0.34 | <0.001 |
| GCS | −0.24 | 0.007 |
| Progesterone | −0.02 | 0.843 |
| Cortisol | 0.23 | 0.012 |
| Progesterone on cortisol | 0.35 | 0.001 |
| Indirect effect | 0.08 | 0.036 |
| FIM-Cog at 6 months | ||
| Age | −0.01 | 0.932 |
| GCS | 0.23 | 0.029 |
| Progesterone | −0.07 | 0.535 |
| Cortisol | −0.34 | 0.002 |
| Progesterone on cortisol | 0.36 | <0.001 |
| Indirect effect | 0.12 | 0.024 |
| FIM-Cog at 12 months | ||
| Age | −0.18 | 0.174 |
| GCS | 0.20 | 0.109 |
| Progesterone | 0.02 | 0.911 |
| Cortisol | −0.26 | 0.035 |
| Progesterone on cortisol | 0.29 | 0.014 |
| Indirect effect | −0.08 | 0.117 |
GOS, Glasgow Outcome Scale; GCS, Glasgow Coma Score; DRS, Disability Rating Scale; FIM-Cog, functional independence measure-cognitive.
Hormone correlations
Table 5 shows intercompartment correlations between mean hormone levels. Correlations between daily hormone values were conducted, and relationships were similar to correlations with averaged values (data not shown) Mean serum and CSF progesterone levels were not significantly correlated among control subjects (r=0.415, p=0.267). The correlation between serum and CSF progesterone levels among TBI subjects, however, was significant (r=0.734, p<0.01), suggesting that after TBI, the contribution of peripheral progesterone to the CNS is substantial. Mean serum and CSF cortisol levels after TBI were also strongly correlated with each other (r=0.662, p<0.01). This relationship is in striking contrast to control levels, which displayed no correlation (r=−0.045; p=0.883), again suggesting that CSF cortisol after TBI is largely derived from the periphery. Table 5 also shows intracompartment correlations between hormones. CSF progesterone and CSF cortisol levels after TBI were strongly correlated (r=0.682, p<0.01), whereas control levels were not correlated (r=0.273; p=0.477). Serum progesterone and serum cortisol were significantly correlated after TBI (r=0.534, p<0.01) and for controls (r=0.748; p<0.05).
Table 5.
Hormone Correlations
| TBI subjects | |||
|---|---|---|---|
| CSF cortisol | Serum cortisol | CSF progesterone | |
| CSF cortisol | ____ | ____ | ____ |
| Serum cortisol | 0.662** | ____ | ____ |
| CSF progesterone | 0.682** | 0.629** | ____ |
| Serum progesterone | 0.397** | 0.534** | 0.734** |
| Control subjects | |||
|---|---|---|---|
| CSF cortisol | Serum cortisol | CSF progesterone | |
| CSF cortisol | ____ | ____ | ____ |
| Serum cortisol | −0.045 | ____ | ____ |
| CSF progesterone | 0.273 | 0.602 | ____ |
| Serum progesterone | −0.412 | 0.748* | 0.415 |
*p<0.05 level (two-tailed); **p<0.01 level (two-tailed).
TBI, traumatic brain injury; CSF, cerebrospinal fluid.
Discussion
Consistent with our hypothesis, CSF cortisol levels were significantly and persistently elevated during the first week after TBI compared with healthy control values, and high CSF cortisol levels were associated with poor outcome across a range of multidimensional outcomes. Specifically, we found that CSF cortisol TRAJ groups with cortisol concentrations higher than control values were associated with worse outcome across multiple domains. TRAJ group categorization models were more predictive of outcome than those where average week 1 hormone levels were explored (data not shown). Similar elevations and effects on outcome have been reported in a variety of neurological conditions, including septic shock, bacterial meningitis, post-traumatic stress disorder, Alzheimer disease, depression, and multiple sclerosis.47–52
This study further extends previous work33,34 by systematically attempting to elucidate the origins and implications behind endogenous hormone profiles after TBI. This goal was accomplished by identifying temporal CSF hormone profiles, investigating correlations between serum and CSF hormone levels, and examining injury-specific relationships between CSF progesterone and cortisol levels.
Both serum and CSF cortisol levels as well as serum and CSF progesterone levels were strongly correlated after TBI relative to controls. This may be because of increased hormone transport from the periphery into the brain resulting from BBB disruption, which is known to occur shortly after TBI. Along with the expected inflammation and stress responses characteristic of this type of injury,33,53,54 a breach in the BBB may explain elevated CSF hormone levels immediately after injury.
After the first few days post-injury, CSF progesterone levels decline to normal levels whereas cortisol levels remain elevated. These findings may be because of the different transport mechanisms that regulate cortisol and progesterone levels in the brain. In addition to passive diffusion, cortisol transport is a highly regulated process that involves specific transport proteins. In particular, overexpression of the transport protein, P-glycoprotein (Pgp) results in the active efflux of cortisol out of the brain through the BBB, a process that is partly responsible for maintaining a healthy CNS environment under physiological conditions.55–57 In fact, multiple studies have shown that Pgp inhibitors significantly increase intracellular cortisol.58,59 Thus, when Pgp expression or activity is disturbed, perhaps by inflammation or other secondary injury cascades, deleterious amounts of cortisol may accumulate in the brain.60,61
Intracerebral progesterone is much less influenced by Pgp activity.62 Instead, free progesterone can readily diffuse across the BBB.63 The initially high CSF progesterone levels and the temporal decline observed with CSF progesterone TRAJ likely reflect temporal changes in BBB permeability associated with severe TBI. Similar temporal declines have been observed with other proteins (e.g., serum S100b) traditionally thought to be highly reflective of BBB integrity.43
In addition to hormone transport kinetics and BBB permeability, injury and inflammation may also reduce the ability of brain cells to metabolize sterol molecules, as is noted in the context of meningitis,48 perhaps leading to further sustained increases in CSF cortisol in the setting of TBI. Moreover, because severe illness and/or chronic stress greatly reduces corticosteroid-binding globulin (CBG) production, there is an increased percentage of free cortisol readily available to cross the BBB and enter the injured brain.47,64 Similar changes in sex hormone binding globulin levels and bioavailable progesterone have not been studied in the context of TBI.
Traditionally, cortisol was thought to be synthesized exclusively from peripheral sources— specifically, the adrenal gland. Recent evidence, however, suggests that both cortisol and progesterone may be synthesized de novo within the brain.65,66 The enzyme 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) regenerates active glucocorticoids from the inert 11-keto forms and is expressed in several organs, including the adult brain.67–69 The enzyme P450scc is also found in the brain, and it mediates the conversion of cholesterol to pregnenolone in glia, which can then be converted to progesterone by 3-β-hydroxysteroid dehydrogenase (3-β–HSD),23 generating a neurosteroid with pleiotropic actions physiologically and after TBI.70,71 Studies have indicated that CNS progesterone levels are transiently elevated in the rat brain after TBI from increased local synthesis of progesterone.72 In addition, upregulation of P450scc has been noted within the spinal cord in response to pain.73 Elevations in endogenous progesterone in CSF may therefore represent the brain's attempt to activate its innate neuroprotective mechanisms; and, as previously noted, peripheral influx from transiently elevated, adrenally derived serum progesterone levels33 may also contribute to this observation.
Endogenous glucocorticoids such as cortisol have long been recognized for their anti-inflammatory effects; however, emerging evidence suggests that during acute brain injury, glucocorticoids may actually exacerbate aspects of inflammation, leading to detrimental effects and damage.74 With some neurological insults, such as trauma, stroke, and seizure, glucocorticoids can increase cytokine activation, thereby exacerbating neurotoxicity and contributing to neuronal loss, particularly of hippocampal neurons.75,76 In fact, a “priming” effect has been noted whereby previous exposure to stress or increased cortisol levels will cause an augmented neuroinflammatory response on second insult.77,78 Studies also show that elevated cortisol levels impair synaptic plasticity and cognition, decrease neurogenesis, and cause dendritic atrophy.79,80 Glucocorticoids can act directly on myeloid cells to increase inflammation and may also indirectly increase inflammation by reducing neurotrophin levels and decreasing expression of proteins that control BBB patency.81,82 Other experimental studies suggest that elevated glucocorticoid levels increase CNS glutamate release, also contributing to neurotoxicity.83
Interestingly, studies have shown that glucocorticoids can have opposing context and time-dependent effects. Specifically, glucocorticoids may have transient anti-inflammatory effects, but as levels remain chronically elevated, cytokine expression is increased and the steroid's effects become pro-inflammatory, as described above.81 Further, evidence suggests that some elevation in cortisol is needed during critical illness and trauma to maintain physiological functions such as systemic blood pressure; however, high-normal cortisol levels observed in critically ill patients may not always imply that they are glucocorticoid sufficient in this regard because cellular responses to glucocorticoids depend both on the amount of hormone it is exposed to and the sensitivity of the glucocorticoid receptor.84 Thus, it will be important to look at both of these factors in future studies when assessing glucocorticoid action. The complex interplay between prolonged cortisol elevations, like those observed in CSF after severe TBI, and inflammatory cytokines may be a fruitful area of future study to begin to determine the mechanistic consequences of prolonged excess endogenous glucocorticoid exposure in the CNS after TBI.
It is also important to recognize that as a precursor to cortisol, peripheral progesterone supplementation may have an effect on endogenous CNS cortisol levels observed after brain injury. As expected, serum cortisol and progesterone are significantly correlated—a phenomenon that we have shown limits serum progesterone as a prognostic indicator for TBI outcomes.33 For this study, we hypothesized that substrate utilization for cortisol production would also limit the capacity of CSF progesterone to serve as a prognostic outcome marker. Indeed, our SEQM models demonstrate that progesterone's relationships with outcome occur indirectly via its relationship with cortisol. Also supporting this finding is the significant association between CSF cortisol and serum progesterone. This CSF hormone relationship is TBI specific, however, suggesting further that BBB dynamics after TBI may allow for significant amounts of peripherally derived progesterone and cortisol to be present in CSF.
Interestingly, age was associated with both outcome and also CSF progesterone and cortisol. Age is also known to augment endogenous serum hormone profiles after TBI.33 Given that CSF cortisol and progesterone profiles are significantly derived from serum production, this association is not surprising. The finding, however, suggests another mechanism by which older age may contribute to secondary injury and poor outcome. Similar to previous work,33 CSF and serum cortisol and progesterone were not different based on sex, suggesting that normal hormone physiology is disrupted after TBI.
The current findings, taken together with existing literature, may help shed light on the possible reasons why previous glucocorticoid trials have failed while ongoing progesterone trials are showing significant promise. If it is true that endogenous cortisol accumulates in the brain because of (1) disrupted transport mechanisms involving efflux out of the brain, (2) an increase in unbound cortisol available for transport through a disrupted BBB, (3) a decrease in CSF cortisol metabolism, and (4) increased de novo synthesis of cortisol in the CNS, then the administration of exogenous glucocorticoids may indeed be detrimental.
The administration of progesterone, on the other hand, may provide benefits in multiple different ways. As previously noted, progesterone is a potent reducer of inflammation, and its neuroprotective effects have been well-established. Importantly, progesterone serves as a precursor for multiple other steroids, and it may be through its metabolites that progesterone exerts its beneficial effects. For example, allopregnanolone is rapidly gaining recognition as a potent neuroprotectant.11 Progesterone is also the precursor for estradiol, which has been extensively characterized for its neuroprotective qualities. While high serum estradiol levels are associated with poor outcome after severe TBI and other populations with significant trauma,33 higher CSF estradiol/testosterone ratios are linked to better outcomes and support the neuroprotection hypothesis for estradiol.34
So while it is possible that supplemental progesterone may be metabolized to cortisol and augment both peripheral and CNS glucocorticoid levels, it is also possible that progesterone supplementation may instead offset any potential negative effects of augmented CNS cortisol with its pleiotropic neuroprotective effects in the CNS and conversion to other hormones with neuroprotective qualities. In fact, it has been recently suggested that progesterone secretion in astrocytes can protect them from corticosterone-induced damage in rats.85 Therefore, it appears that there are multiple indirect pathways by which progesterone exerts its beneficial effects, and it is possible that on administration, the utilization, transport, and metabolism of progesterone may be uniquely tailored to each person's complex biological environment such that it serves multiple contextually dependent neuroprotective roles.
While this study is novel in its presentation of CSF hormone relationships, there are some limitations. We only measured total hormone levels for each of the hormones evaluated in this study. Some literature suggests that the biologically active free fraction of cortisol and CBG concentrations change in response to acquired brain injury without notable deviations outside of the reference range for total hormone levels.86 Further, hormones were measured in the CSF and not directly in the brain interstitial fluid. Studies suggest, however, that CSF measurements are a suitable surrogate for assessing free drug concentrations in the brain.87–89 Lastly, like all observational studies, the findings may be subject to bias and confounding effects.
Future directions include further exploration of the chronic effects of prolonged cortisol exposure, including depression and hypogonadism. Acute cortisol associations with inflammation, glial scarring, and post-traumatic epilepsy development could be studied as potential mediators of these chronic effects. In addition, assessing injury effects on synthetic/metabolic hormone enzyme activity may be informative. Last, it may be of interest to consider genetic studies for glucocorticoid receptors and Pgps as potential moderators of cortisol effects in the CNS after injury.
Acknowledgments
The authors would like to thank Tammy Loucks, Ph D., for her assistance with hormone measurements. This work was supported by the Centers for Disease Control and Prevention (R49 CCR323155), the Department of Defense (W81XWH-071-0701), and the National Institutes of Health (5P01NS030318).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Roof R.L., Duvdevani R., and Stein D.G. (1992). Progesterone treatment attenuates brain edema following contusion injury in male and female rats. Restor. Neurol. Neurosci. 4, 425–427 [DOI] [PubMed] [Google Scholar]
- 2.Roof R.L., Duvdevani R., and Stein D. G. (1993). Gender influences outcome of brain injury: progesterone plays a protective role. Brain Res. 607, 333–336 [DOI] [PubMed] [Google Scholar]
- 3.Schumacher M., Akwa Y., Guennoun R., Robert F., Labombarda F., Desarnaud F., Robel P., De Nicola A. F., and Baulieu E.E. (2000). Steroid synthesis and metabolism in the nervous system: trophic and protective effects. J. Neurocytol. 29, 307–326 [DOI] [PubMed] [Google Scholar]
- 4.Brinton R.D., Thompson R.F., Foy M.R., Baudry M., Wang J., Finch C.E., Morgan T.E., Pike C.J., Mack W.J., Stanczyk F.Z., and Nilsen J. (2008). Progesterone receptors: form and function in brain. Front Neuroendocrinol. 29, 313–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meffre D., Labombarda F., Delespierre B., Chastre A., De Nicola A.F., Stein D.G., Schumacher M., and Guennoun R. (2013). Distribution of membrane progesterone receptor alpha in the male mouse and rat brain and its regulation after traumatic brain injury. Neuroscience 231, 111–124 [DOI] [PubMed] [Google Scholar]
- 6.Sayeed I., and Stein D.G. (2009). Progesterone as a neuroprotective factor in traumatic and ischemic brain injury. Prog. Brain Res. 175, 219–237 [DOI] [PubMed] [Google Scholar]
- 7.Stein D.G., and Wright D.W. (2010). Progesterone in the clinical treatment of acute traumatic brain injury. Expert Opin. Investig. Drugs 19, 847–857 [DOI] [PubMed] [Google Scholar]
- 8.Stein D.G. (2011). Is Progesterone a worthy candidate as a novel therapy for traumatic brain injury? Dialogues Clin. Neurosci. 13, 352–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright D.W., Kellermann A.L., Hertzberg V.S., Clark P.L., Frankel M., Goldstein F.C., Salomone J.P., Dent L.L., Harris O.A., Ander D.S., Lowery D.W., Patel M.M., Denson D.D., Gordon A.B., Wald M.M., Gupta S., Hoffman S.W., and Stein D.G. (2007). ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann. Emerg. Med. 49, 391–402 [DOI] [PubMed] [Google Scholar]
- 10.Djebaili M., Hoffman S.W., and Stein D.G. (2004). Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat pre-frontal cortex. Neuroscience 123, 349–359 [DOI] [PubMed] [Google Scholar]
- 11.He J., Hoffman S.W., and Stein D.G. (2004). Allopregnanolone, a progesterone metabolite, enhances behavioral recovery and decreases neuronal loss after traumatic brain injury. Restor. Neurol. Neurosci. 22, 19–31 [PubMed] [Google Scholar]
- 12.Cooke P.S., Nanjappa M.K., Yang Z., and Wang K.K. (2013). Therapeutic effects of progesterone and its metabolites in traumatic brain injury may involve non-classical signaling mechanisms. Front. Neurosci. 7, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao G., Wei J., Yan W., Wang W., and Lu Z. (2008). Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Crit. Care. 12, R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bracken M.B., Shepard M.J., Collins W.F., Holford T.R., Young W., Baskin D.S., Eisenberg H.M., Flamm E., Leo-Summers L., Maroon J., et al. (1990). A randomized controlled trial of methylprednisolone or naloxone in the treatment of acute spinal cord injury. Results of the Second National Acute Spinal Cord Injury Study N. Eng. J. Med. 322, 1405–1411 [DOI] [PubMed] [Google Scholar]
- 15.Bracken M.B., Shepard M.J., Collins W.F., Jr., Holford T.R., Baskin D.S., Eisenberg H.M., Flamm E., Leo-Summers L., Maroon J.C., Marshall L.F., et al. (1992). Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. Results of the Second National Acute Spinal Cord Injury Study. J Neurosurg. 76, 23–31 [DOI] [PubMed] [Google Scholar]
- 16.Bracken M.B., Shepard M.J., Holford T.R., Leo-Summers L., Aldrich E.F., Fazl M., Fehlings M., Herr D.L., Hitchon P.W., Marshall L.F., Nockels R.P., Pascale V., Perot P.L., Jr., Piepmeier J., Sonntag V.K., Wagner F., Wilberger J.E., Winn H.R., and Young W. (1997). Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA 277, 1597–1604 [PubMed] [Google Scholar]
- 17.Faupel G., Reulen H.J., Müller D., and Schürmann K. (1976). Double-blind study on the effects of steroids on severe closed head injury, in: Dynamics of Brain Edema. Pappius M.M., and Feindel W. (eds). Springer-Verlag: Berlin, pps. 337–343 [Google Scholar]
- 18.Beauchamp K., Mutlak H., Smith W.R., Shohami E., and Stahel P.F. (2008). Pharmacology of traumatic brain injury: where is the “golden bullet”? Mol. Med. 14, 731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alderson P., and Roberts I. (2005). Corticosteroids for acute traumatic brain injury. Cochrane Database Sys. Rev. 1, CD000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galicich J.H., and French L.A. (1961). Use of dexamethasone in the treatment of cerebral edema resulting from brain tumors and brain surgery. Am. Pract. Dig. Treat. 12, 169–174 [PubMed] [Google Scholar]
- 21.Lamontagne F., Quiroz Martinez H., Adhikari N.K., Cook D.J., Koo K.K., Lauzier F., Turgeon A.F., Kho M.E., Burns K.E., Chant C., Fowler R., Douglas I., Poulin Y., Choong K., Ferguson N.D, and Meade M.O. (2013). Corticosteroid use in the intensive care unit: a survey of intensivists. Can. J. Anaesth. 60, 652–659 [DOI] [PubMed] [Google Scholar]
- 22.Roberts I., Yates D., Sandercock P., Farrell B., Wasserberg J., Lomas G., Cottingham R., Svoboda P., Brayley N., Mazairac G., Laloë V., Muñoz-Sánchez A., Arango M., Hartzenberg B., Khamis H., Yutthakasemsunt S., Komolafe E., Olldashi F., Yadav Y., Murillo-Cabezas F., Shakur H., Edwards P., and CRASH trial collaborators (2004). Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomized placebo-controlled trial. Lancet 364, 1321–1328 [DOI] [PubMed] [Google Scholar]
- 23.Schumacher M., Hussain R., Gago N., Oudinet J.P., Mattern C., and Ghoumari A.M. (2012). Progesterone synthesis in the nervous system: implications for myelination and myelin repair. Front. Neurosci. 6, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baulieu E.E., Robel P., and Schumacher M. (2001). Neurosteroids: beginning of the story. Int. Rev. Neurobiol. 46, 1–32 [DOI] [PubMed] [Google Scholar]
- 25.Heffelfinger A.K., and Newcomer J.W. (2001). Glucocorticoid effects on memory function over the human life span. Dev. Psychopathol. 13, 491–513 [DOI] [PubMed] [Google Scholar]
- 26.De Kloet E.R. (2004). Hormones and the stressed brain. Ann. N. Y. Acad. Sci. 1018, 1–15 [DOI] [PubMed] [Google Scholar]
- 27.Lupien S.J., Maheu F., Tu M., Fiocco A., and Schramek T.E. (2007). The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain Cogn. 65, 209–237 [DOI] [PubMed] [Google Scholar]
- 28.Swaab D.F., Bao A.M., and Lucassen P.J. (2005). The stress system in the human brain in depression and neurodegeneration. Ageing Res. Rev. 4, 141–194 [DOI] [PubMed] [Google Scholar]
- 29.Kling S.R., Manna P.R., Ishii T., Syapin P.J., Ginsberg S.D., Wilson K., Walsh L.P., Parker K.L., Stocco D.M., Smith R.G., and Lamb D.J. (2002). An essential component in steroid synthesis, the steroidogenic acute regulatory protein, is expressed in discrete regions of the brain. J. Neurosci. 22, 10613–10620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu L., Romero D.G., Gomez-Sanchez C.E., and Gomez-Sanchez E.P. (2002). Steroidogenic enzyme gene expression in the human brain. Mol. Cell. Endocrinol. 190, 9–17 [DOI] [PubMed] [Google Scholar]
- 31.Stoffel-Wagner B. (2003). Neurosteroid biosynthesis in the human brain and its clinical implications. Ann. N.Y. Acad. Sci. 1007, 64–78 [DOI] [PubMed] [Google Scholar]
- 32.Pluchino N., Santoro A., Casarosa E., Wenger J.M., Genazzani A.D., Petignat P., and Genazzani A.R. (2013). Advances in neurosteroids: role in clinical practice. Climacteric 16, Suppl 1, 8–17 [DOI] [PubMed] [Google Scholar]
- 33.Wagner A.K., McCullough E.H., Niyonkuru C., Ozawa H., Loucks T., Dobos J.A., Brett C.A., Santarsieri M., Dixon C.E., Berga S., and Fabio A. (2011). Acute serum hormone levels: characterization and prognosis after severe traumatic brain injury. J. Neurotrauma 28, 871–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garringer J.A., Niyonkuru C., McCullough E.H., Loucks T., Dixon C.E., Conley Y.P., Berga S., and Wagner A.K. (2013). Impact of aromatase genetic variation on hormone levels and global outcome after severe TBI. J. Neurotrauma 30, 1415–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brain Trauma Foundation, American Association of Neurological Surgeons, and Congress of Neurological Surgeons (2007). Guidelines for the management of severe traumatic brain injury. J. Neurotrauma 24, Suppl 1, S1–S106 [DOI] [PubMed] [Google Scholar]
- 36.Zafonte R., Friedewald W.T., Lee S.M., Levin B., Diaz-Arrastia R., Ansel B., Eisenberg H., Timmons S.D., Temkin N., Novack T., Ricker J., Merchant R., and Jallo J. (2009). The citicoline brain injury treatment (COBRIT) trial: design and methods. J. Neurotrauma 26, 2207–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jennett B., and Bond M. (1975). Assessment of outcome after severe brain damage. Lancet 1, 480–484 [DOI] [PubMed] [Google Scholar]
- 38.Rappaport M., Hall K.M., Hopkins K., Belleza T., and Cope D.N. (1982). Disability rating scale for severe head trauma: coma to community. Arch. Phys. Med. Rehabil. 63, 118–123 [PubMed] [Google Scholar]
- 39.Hamilton B.B., Granger C.V., Sherwin F.S., Zielezny M., and Tashman J.S. (1987). A uniform national data system for medical rehabilitation, in: Rehabilitation Outcomes: Analysis and Measurement. Fuhrer M. (ed). Brookes: Baltimore, pps.137–147 [Google Scholar]
- 40.Brown A.W., Malec J.F., McClelland R.L., Diehl N.N., Englander J., and Cifu D.X. (2005). Clinical elements that predict outcome after traumatic brain injury: a prospective multicenter recursive partitioning (decision-tree) analysis. J. Neurotrauma 22, 1040–1051 [DOI] [PubMed] [Google Scholar]
- 41.Andelic N., Sigurdardottir S., Schanke A.K., Sandvik L., Sveen U., and Roe C. (2010). Disability, physical health and mental health 1 year after traumatic brain injury. Disabil. Rehabil. 32, 1122–1131 [DOI] [PubMed] [Google Scholar]
- 42.Barnes C., Conner D., Legault L., Reznickova N., and Harrison-Felix C. (2004). Rehabilitation outcomes in cognitively impaired patients admitted to skilled nursing facilities from the community. Arch. Phys. Med. Rehabil. 85, 1602–1607 [DOI] [PubMed] [Google Scholar]
- 43.Goyal A., Failla M.D., Niyonkuru C., Amin K., Fabio A., Berger R.P., and Wagner A.K. (2013). S100b as a prognostic biomarker in outcome prediction for patients with severe traumatic brain injury. J. Neurotrauma 30, 946–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niyonkuru C., Wagner A.K., Ozawa H., Amin K., Goyal A., and Fabio A. (2013). Group-based trajectory analysis applications for prognostic biomarker model development in severe TBI: a practical example. J. Neurotrauma 30, 938–945 [DOI] [PubMed] [Google Scholar]
- 45.Nagin D.S. (2005). Group-based Modeling of Development. Harvard University Press: Cambridge [Google Scholar]
- 46.Jones B.L., Nagin D., and Roeder K. (2001). A SAS procedure based on mixture models for estimating developmental trajectories. Sociol. Meth. Res. 29, 374–393 [Google Scholar]
- 47.Ho J.T., Al-Musalhi H., Chapman M.J., Quach T., Thomas P.D., Bagley C.J., Lewis J.G., and Torpy D.J. (2006). Septic shock and sepsis: a comparison of total and free plasma cortisol levels. J. Clin. Endocrinol. Metab. 91, 105–114 [DOI] [PubMed] [Google Scholar]
- 48.Holub M., Beran O., Dzupova O., Hnykova J., Lacinova Z., Prihodova J., Prochazka B., and Helcl M. (2007). Cortisol levels in cerebrospinal fluid correlate with severity and bacterial origin of meningitis. Crit. Care 11, R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baker D.G., Ekhator N.N., Kasckow J.W., Dashevsky B., Horn P.S., Bednarik L., and Geracioti T.D., Jr (2005). Higher levels of basal serial CSF cortisol in combat veterans with posttraumatic stress disorder. Am. J. Psychiatry 162, 992–994 [DOI] [PubMed] [Google Scholar]
- 50.Czech C., Berndt P., Busch K., Schmitz O., Wiemer J., Most V., Hampel H., Kastler J., and Senn H. (2012). Metabolite profiling of Alzheimer's disease cerebrospinal fluid. PLoS One 7(2), e31501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerner R.H., and Wilkins J.N. (1983). CSF cortisol in patients with depression, mania, or anorexia nervosa and in normal subjects. Am. J. Psychiatry 140, 92–94 [DOI] [PubMed] [Google Scholar]
- 52.Erkut Z.A., Endert E., Huitinga I., and Swaab D.F. (2002). Cortisol is increased in postmortem cerebrospinal fluid of multiple sclerosis patients: relationship with cytokines and sepsis. Mult. Scler. 8, 229–236 [DOI] [PubMed] [Google Scholar]
- 53.Boles J.A., Goyal A., and Wagner A.K. (2013). Chronic inflammation after severe traumatic brain injury: characterization and associations with outcome. J. Neurotrauma 30, A-1–A-183 [DOI] [PubMed] [Google Scholar]
- 54.Boles J.A., Amin K., Niyonkuru C., Berger R.P., Kochanek P.M., Tisherman S., and Wagner A.K. (2012). The influence of isolated head injury status on inflammation cytokine levels post traumatic brain injury [abstract]. J. Neurotrauma 29, A35 [Google Scholar]
- 55.Van Kalken C.K., Broxterman H.J., Pinedo H.M., Feller N., Dekker H., Lankelma J., and Giaccone G. (1993). Cortisol is transported by the multidrug resistance gene product P-glycoprotein. Br. J. Cancer. 67, 284–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schinkel A.H. (1999). P-Glycoprotein, a gatekeeper in the blood-brain barrier. Adv. Drug Deliv. Rev. 36, 179–194 [DOI] [PubMed] [Google Scholar]
- 57.Loscher W., and Potschka H. (2005). Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx. 2, 86–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karssen A.M., Meijer O.C., van der Sandt I.C., Lucassen P.J., de Lange E.C., de Boer A.G., and de Kloet E.R. (2001). Multidrug resistance P-glycoprotein hampers the access of cortisol but not of corticosterone to mouse and human brain. Endocrinology 142, 2686–2694 [DOI] [PubMed] [Google Scholar]
- 59.Farrell R.J., Menconi M.J., Keates A.C., and Kelly C.P. (2002). P-Glycoprotein-170 inhibition significantly reduces cortisol and ciclosporin efflux from human intestinal epithelial cells and T lymphocytes. Aliment. Pharmacol. Ther. 16, 1021–1031 [DOI] [PubMed] [Google Scholar]
- 60.Hartz A.M., Bauer B., Fricker G., and Miller D.S. (2006). Rapid modulation of P-glycoprotein-mediated transport at the blood-brain barrier by tumor necrosis factor-alpha and lipopolysaccharide. Mol. Pharmacol. 69, 462–470 [DOI] [PubMed] [Google Scholar]
- 61.Roberts D.J. and Goralski K.B. (2008). A critical overview of the influence of inflammation and infection on P-glycoprotein expression and activity in the brain. Expert Opin. Drug Metab. Toxicol. 4, 1245–1264 [DOI] [PubMed] [Google Scholar]
- 62.Uhr M., Holsboer F., and Muller M.B. (2002). Penetration of endogenous steroid hormones corticosterone, cortisol, aldosterone and progesterone into the brain is enhanced in mice deficient for both mdr1a and mdr1b P-glycoproteins. J. Neuroendocrinol. 14, 753–759 [DOI] [PubMed] [Google Scholar]
- 63.Pardridge W.M., and Mietus L.J. (1979). Transport of steroid hormones through the rat blood-brain barrier. Primary role of albumin-bound hormone. J. Clin. Invest. 64, 145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Torpy D.J., and Ho J.T. (2007). Value of free cortisol measurement in systemic infection. Horm. Metab. Res. 39, 439–444 [DOI] [PubMed] [Google Scholar]
- 65.Strömstedt M., and Waterman M.R. (1995). Messenger RNAs encoding steroidogenic enzymes are expressed in rodent brain. Brain Res. Mol. Brain Res. 34, 75–88 [DOI] [PubMed] [Google Scholar]
- 66.Mellon S.H., and Deschepper C.F. (1993). Neurosteroid biosynthesis: genes for adrenal steroidogenic enzymes are expressed in the brain. Brain Res. 629, 283–292 [DOI] [PubMed] [Google Scholar]
- 67.Chapman K., Holmes M. and Seckl J. (2013). 11β-Hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol. Rev. 93, 1139–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wyrwoll C.S., Holmes M.C., and Seckl J.R. (2011). 11β-Hydroxysteroid dehydrogenases and the brain: from zero to hero, a decade of progress. Front. Neuroendocrinol. 32, 265–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.MacKenzie S.M., Clark C.J., Fraser R., Gomez-Sanchez C.E., Connell J.M., and Davies E. (2000). Expression of 11β hydroxylase and aldosterone synthesis genes in the rat brain. J. Mol. Endocrinol. 24, 321–328 [DOI] [PubMed] [Google Scholar]
- 70.Frye C.A. (2009). Neurosteroids' effects and mechanisms for social, cognitive, emotional, and physical functions. Psychoneuroendocrinology 34, Suppl 1, S143–S161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stein D.G. (2011). Progesterone in the treatment of acute traumatic brain injury: a clinical perspective and update. Neuroscience 191, 101–106 [DOI] [PubMed] [Google Scholar]
- 72.Meffre D., Delespierre B., Gouézou M., Schumacher M., Stein D.G., and Guennoun R. (2007). 3beta-Hydroxysteroid dehydrogenase/5-ene-4-ene isomerase mRNA expression in rat brain: effect of pseudopregnancy and traumatic brain injury. J. Steroid Biochem. Mol. Biol. 104, 293–300 [DOI] [PubMed] [Google Scholar]
- 73.Patte-Mensah C., Li S., and Mensah-Nyagan A.G. (2004). Impact of neuropathic pain on the gene expression and activity of cytochrome P450side-chain-cleavage in sensory neural networks. Cell Mol. Life Sci. 61, 2274–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sorrells S.F., Caso J.R., Munhoz C.D., and Sapolsky R.M. (2009). The stressed CNS: when glucocorticoids aggravate inflammation. Neuron 64, 33–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caso J.R., Moro M.A., Lorenzo P., Lizasoain I., and Leza J.C. (2007).Involvement of IL-1beta in acute stress-induced worsening of cerebral ischaemia in rats. Eur Neuropsychopharmacol. 17, 600–607 [DOI] [PubMed] [Google Scholar]
- 76.Sapolsky R.M., and Pulsinelli W.A. (1985). Glucocorticoids potentiate ischemic injury to neurons: therapeutic implications. Science 229, 1397–1400 [DOI] [PubMed] [Google Scholar]
- 77.Barber A.E., Coyle S.M., Marano M.A., Fischer E., Calvano S.E., Fong Y., Moldawer L.L., and Lowry S.F. (1993). Glucocorticoid therapy alters hormonal and cytokine responses to endotoxin in man. J. Immunol. 150, 1999–2006 [PubMed] [Google Scholar]
- 78.Johnson J.D., O'Connor K.A., Deak T., Stark M., Watkins L.R., and Maier S.F. (2002). Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav.Immun. 16, 461–476 [DOI] [PubMed] [Google Scholar]
- 79.Lupien S.J., and McEwen B.S. (1997). The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res. Brain Res. Rev. 24, 1–27 [DOI] [PubMed] [Google Scholar]
- 80.McEwen B.S., and Magarinos A.M. (2001). Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum. Psychopharmacol. 16, S7–S19 [DOI] [PubMed] [Google Scholar]
- 81.Dinkel K., MacPherson A., and Sapolsky R.M. (2003). Novel glucocorticoid effects on acute inflammation in the CNS. J. Neurochem. 84, 705–716 [DOI] [PubMed] [Google Scholar]
- 82.Sorrells S.F., Caso J.R., Munhoz C.D., Hu C.K., Tran K.V., Miguel Z.D., Chien B.Y., and Sapolsky R.M. (2013). Glucocorticoid signaling in myeloid cells worsens acute CNS injury and inflammation. J. Neurosci. 33, 7877–7889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Popoli M., Yan Z., McEwen B.S., and Sanacora G. (2011). The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 13, 22–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bamberger C.M., Schulte H.M., and Chrousos G.P. (1996). Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocr. Rev. 17, 245–261 [DOI] [PubMed] [Google Scholar]
- 85.Guo W.Z., Miao Y.L., An L.N., Wang X.Y., Pan N.L., Ma Y.Q., Chen H.X., Zhao N., Zhang H., Li Y.F., and Mi W.D. (2013). Midazolam provides cytoprotective effect during corticosterone-induced damages in rat astrocytes by stimulating steroidogenesis. Neurosci. Lett. 547, 53–58 [DOI] [PubMed] [Google Scholar]
- 86.Savaridas T., Andrews P.J., and Harris B. (2004). Cortisol dynamics following acute severe brain injury. Intensive Care Med. 30, 1479–1483 [DOI] [PubMed] [Google Scholar]
- 87.Lin J.H. (2008). CSF as a surrogate for assessing CNS exposure: an industrial perspective. Curr. Drug Metab. 9, 46–59 [DOI] [PubMed] [Google Scholar]
- 88.Liu X., Smith B.J., Chen C., Callegari E., Becker S.L., Chen X., Cianfrogna J., Doran A.C., Doran S.D., Gibbs J.P., Hosea N., Liu J., Nelson F.R., Szewc M.A., and Van Deusen J. (2006) Evaluation of cerebrospinal fluid concentration and plasma free concentration as a surrogate measurement for brain free concentration. Drug Metab. Dispos. 34, 1443–1447 [DOI] [PubMed] [Google Scholar]
- 89.Liu X., Van Natta K., Yeo H., Vilenski O., Weller P.E., Worboys P.D., and Monshouwer M. (2009). Unbound drug concentration in brain homogenate and cerebral spinal fluid at steady state as a surrogate for unbound concentration in brain interstitial fluid. Drug Metab. Dispos. 37, 787–793 [DOI] [PubMed] [Google Scholar]