Table 1.
Radioligand Competition Binding Affinity(Ki)Data of PAM Derivatives
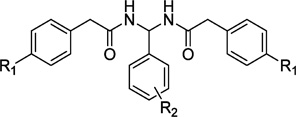 | |||||||
|---|---|---|---|---|---|---|---|
| compd | R1 | R2 | MW | cLogP | Ki (CB2), nMa,b | Ki (CB1, nMa,c | SId |
| 9 | H | p-(CH3)2N- | 401.50 | 4.04 | 777 | >20000 | >26 |
| 11 | H | H- | 358.43 | 3.93 | 9930 | NT | |
| 12 | H | o-F- | 376.42 | 4.08 | 35330 | NT | |
| 13 | H | m-F- | 376.42 | 4.08 | 12670 | NT | |
| 14 | H | p-F- | 376.42 | 4.08 | 10900 | NT | |
| 15 | H | p-Cl- | 392.88 | 4.54 | 3081 | NT | |
| 16 | H | p-Br- | 437.33 | 4.70 | 2226 | NT | |
| 17 | H | p-CH3- | 372.46 | 4.45 | 494 | 109 | |
| 18 | H | p-i-C3H7- | 400.51 | 5.18 | 85 | >20000 | >235 |
| 19 | H | p-CH3O- | 388.46 | 3.78 | 783 | >20000 | >26 |
| 20 | H | p-C2HsO- | 402.49 | 4.13 | 1500 | NT | |
| 21 | H | p-i-C3H7O- | 416.51 | 4.55 | 313 | >20000 | >64 |
| 22 | H | o-CF3- | 426.43 | 4.81 | 11780 | NT | |
| 23 | H | p-CF3- | 426.43 | 4.81 | 596 | >20000 | >34 |
| 24 | H | p-NO2 | 403.43 | 3.87 | NB | NT | |
| 25 | H | p-H2N- | 373.45 | 2.51 | 12550 | NT | |
| 26 | H | p-(C2H5)2N- | 429.55 | 4.76 | 64 | >20000 | >313 |
| 27 | H | p-(C3H7)2N- | 457.61 | 5.80 | 22 | >20000 | >909 |
| 28 | H | p-(C4H9)2N- | 485.66 | 6.69 | 221 | >20000 | >90 |
| 29 | H | p-(benzyl)2N- | 553.69 | 7.33 | 203 | >20000 | >99 |
| 30 | H | p-pyrrolidinyl- | 427.53 | 4.45 | 71 | >20000 | >281 |
| 31 | H | p-piperidyl- | 441.56 | 4.89 | 595 | >20000 | >34 |
| 32 | Cl | H- | 427.32 | 5.14 | NB | NT | |
| 33 | Cl | o-F- | 445.31 | 5.29 | 10850 | NT | |
| 34 | Cl | p-F- | 445.31 | 5.29 | NB | NT | |
| 35 | Cl | p-Cl- | 461.77 | 5.75 | 154 | >20000 | >130 |
| 36 | Cl | p-CH3- | 441.35 | 5.66 | 462 | >20000 | >43 |
| 37 | Cl | p-CH3O- | 457.35 | 4.98 | 310 | >20000 | >65 |
| 38 | Cl | o-CF3- | 495.32 | 6.02 | 158 | >20000 | >127 |
| 39 | Cl | p-CF3- | 495.32 | 6.02 | 101 | >20000 | >198 |
| 40 | Cl | p-NO2- | 472.32 | 5.08 | NB | NT | |
| 41 | CF3 | H- | 494.43 | 5.69 | NB | NT | |
| 42 | CF3 | o-F- | 512.42 | 5.83 | NB | NT | |
| 43 | CF3 | p-F- | 512.42 | 5.83 | NB | NT | |
| 44 | CF3 | p-Cl- | 528.87 | 6.29 | NB | NT | |
| 45 | CF3 | p-CH3- | 508.46 | 6.20 | NB | NT | |
| 46 | CF3 | p-CH3O- | 524.45 | 5.53 | NB | NT | |
| 47 | CF3 | p-CF3- | 562.43 | 6.57 | NB | NT | |
| 1e,f | 2.1 | NT | |||||
| 10e,g | NT | 10.6 | |||||
Binding affinities of compounds for CB1 and CB2 receptor were evaluated using [3H]CP-55,940 radioligand competition binding assay.
NB: no binding, Ki > 20000 nM.
NT: not tested.
SI: selectivity index for CB2, calculated as Ki(CB1)/Ki(CB2) ratio.
The binding affinities of reference compounds were evaluated in parallel with compounds 9, 11–61 under the same conditions.
CB2 reference compound SR144528.
CB1 reference compound SR141716.
