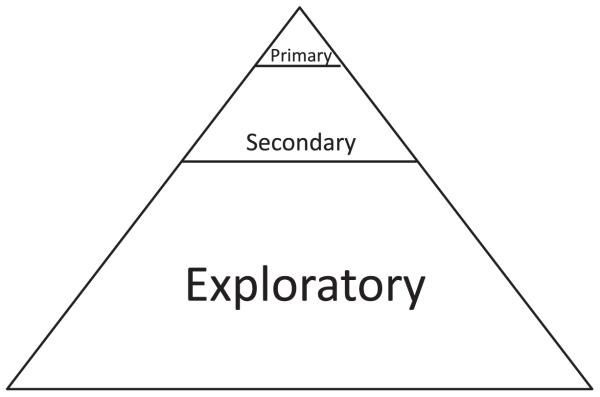
Figure 2.
The traditional end-point hierarchy for end points in a phase III clinical trial. A small number of primary end points with multiplicity error correction are supported by a larger number of secondary end points. The largest number of end points are non-prospectively declared exploratory end points.