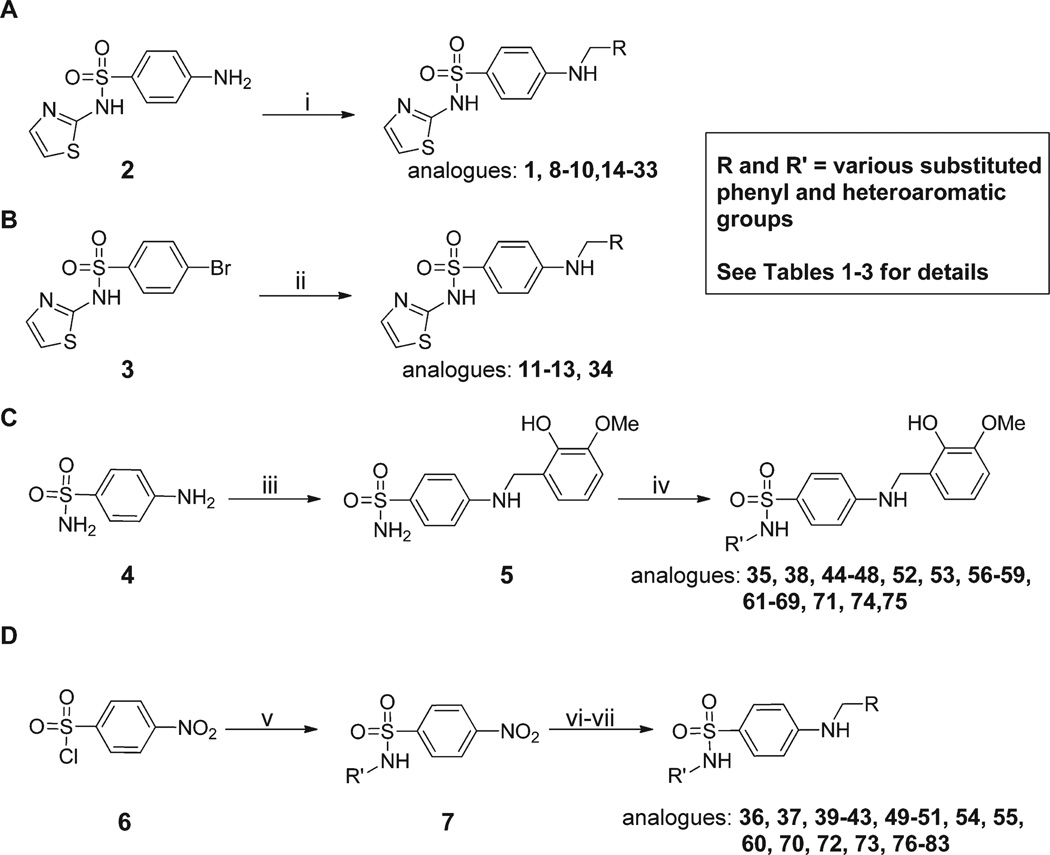
Scheme 1.
Synthesis of Analogues 1–83a
aReagents and conditions: (i) RCHO (1.5 equiv), EtOH, 3–18 h, reflux, NaBH4 (2.0 equiv), 0.5–0.6 h at rt; (ii) RCH2NH2 (1.2 equiv), Xantphos (0.06 equiv), Pd2dba3 (0.02 equiv), NaOtBu (2.5 equiv), 1,4-dioxane, MW, 30 min, 100 °C; (iii) 2-hydroxy-3-methoxybenzaldehyde (1.2 equiv), EtOH, 6 h, reflux, NaBH4 (1.5 equiv), 30 min, rt, 95%; (iv) R′Br (1.2 equiv), N,N′-dimethylethylenediamine, CuI (0.05 equiv), K2CO3 (2.5 equiv), 80 °C, 6–8 h; (v) R′NH2, pyridine, 100 °C, 1.5–18 h; (vi) 10% Pd/C, MeOH/EtOAc/THF (1:1:1), 50 bar, 50 °C or zinc (4.0 equiv), AcOH (4.0 equiv), methanol, 60 °C, 30 min−2 h; (vii) 2-hydroxy-R-benzaldehyde (1.2 equiv), EtOH, 18 h, reflux, NaBH4 (3.0 equiv).