Abstract
Among infants exposed to human immunodeficiency virus type 1 (HIV-1), detection of viral infection at birth was increased by 39% (95% confidence interval, 19%–47%) by increasing DNA input from dried blood spots into polymerase chain reaction. Infants with low concentrations of HIV-1 at birth may be the best target population to evaluate whether immediate antiretroviral therapy can prevent long-term infection.
Keywords: HIV-1, infant diagnosis, HIV-1 DNA, mother-to-child transmission, HIV-1 cure
The recent report of a human immunodeficiency virus type 1 (HIV-1)–infected infant who appears to have been cured of HIV-1 following antiretroviral therapy (ART) initiated within hours of birth has raised hopes for complete prevention of perinatal transmission of HIV-1 [1]. The “Mississippi baby” is hypothesized to have been treated sufficiently early in the course of infection that the virus had not yet established a long-lived infectious reservoir in CD4+ T cells. Conceivably, ART prevented infection of additional cells, so that decay of infected cells cured the infant.
For ART to prevent establishment of an active viral reservoir, it most likely needs to be initiated very early in infection, as in the Mississippi baby and similar cases among adults in the Visconti cohort [2]. In the absence of antiretroviral prophylaxis, an estimated 33% of all perinatal HIV-1 transmissions occur in the late third trimester or during labor or delivery [3, 4]. In pregnancies without maternal ART prophylaxis, or where ART prophylaxis has failed, infants infected during this time frame may be ideal candidates for early ART, as used in the Mississippi baby.
In the absence of maternal ART, infant infection can be diagnosed by testing plasma HIV-1 RNA. However, suppression of HIV-1 replication by maternal ART requires infant diagnostic testing that targets HIV-1 DNA. Dried blood spot (DBS) testing collected on filter paper is used throughout the world for infant diagnosis. The sensitivity of HIV-1 DNA detection in DBS testing is variable, with false negatives due to low copies of viral DNA, low blood volumes [5, 6], or inhibitors of polymerase chain reaction (PCR) [7]. Even highly sensitive assays that test for both HIV-1 RNA and DNA have a lower sensitivity at birth vs at 6 weeks of life [8].
We used sensitive methods to test a cohort of HIV-1–exposed Mozambican infants at the time of birth to determine the prevalence of HIV-1 infections with low concentrations of viral DNA that might be missed by routine diagnostic testing. These infants are likely to have early infection, which could potentially be cured with early, aggressive ART.
METHODS
Study Design
HIV-1–exposed Mozambican infants who received single-dose nevirapine (sdNVP) prophylaxis were eligible to enroll in an observational study of reservoirs of drug-resistant HIV-1 [9]. Clinical data and DBS samples collected at birth, 2, 4, 6, and 8 weeks of life were sent weekly via express mail to Seattle, Washington, for HIV-1 diagnostic testing.
Collection and Testing of DBS Specimens for HIV-1 DNA
Whole blood was spotted on FTA classic filter paper cards (Whatman Bioscience, Florham Park, New Jersey). Initial testing was performed by nested PCR of the HIV-1 DNA pol region using a single 3-mm diameter DBS punch [9–11], which, given a DBS diameter of 25.4 mm and capacity for approximately 200 µL of blood, contained approximately 3 µL of blood. One positive test result was interpreted as “possible HIV-1 infection,” with diagnosis confirmed by testing a subsequent specimen, or, if unavailable, a separate aliquot of blood from the birth DBS sample.
To assess the prevalence of HIV-1 infection at birth with “low” HIV-1 DNA concentration, defined as below the sensitivity of our previous assay [9], a larger volume of the infant's birth DBS (20 punches, equivalent to 60 µL of whole blood) was tested from those infants testing negative at birth, but positive by 8 weeks of life, or those lost to follow-up after 2 weeks of age. Infants testing negative for HIV-1 DNA at 4–8 weeks of age were presumed to be HIV-1 uninfected at birth, and were not retested. PCR amplicons were sequenced to evaluate genetic linkage between birth and later specimens (or second amplicon from birth DBS) by phylogenetic analysis and exclude laboratory contamination.
HIV-1 DNA Quantification
The HIV-1 DNA in infants’ specimens was quantified using a real-time quantitative nested PCR (qPCR) of gag [9]. Three DBS punches, totaling 9 µL of whole blood, were tested in triplicate (1 DBS punch per reaction). The qPCR of the β-globin gene was performed to calculate the number of nucleated cells submitted to PCR [9], to derive the number of copies of HIV-1 DNA/106 cells. In addition, the proportion of positive HIV-1 pol DNA PCR out of 20 reactions per specimen was used to roughly estimate the viral concentration. Given a median of 24 067 nucleated cells per DBS punch, the sampling of 20 DBS punches using a nested PCR sensitive to single-copy detection theoretically improved our limited of detection to approximately 2 copies of HIV-1 DNA/106 cells. Sequencing of the amplified HIV-1 DNA with phylogenetic linkage of each infant's two amplicons was required to define a HIV-1 DNA–positive DBS, as we acknowledge false-positive assays could misdiagnose infants as HIV-1 infected. The proportion of positive HIV-1 pol PCR from each infant's DBS was tabulated (number of positive PCR/20 reactions/DBS).
RESULTS
A total of 849 HIV-1–exposed infants who received sdNVP prophylaxis were enrolled between June 2005 and June 2008 and had a blood sample collected at birth. In the initial test of a single 3-µL aliquot, 47 of 849 infants (6%) tested positive for HIV-1 DNA at birth. After retesting, 15 additional infants were identified, for a total of 62 of 849 infants (7%). Across the 412 infants’ DBSs retested using 20 punches in 20 separate PCRs per DBS, a median of 20 (range, 1–20) was positive per DBS. The range of nucleated cells measured by β-globin PCR was 7533–94 700 per punch (median 24 067; interquartile range, 14 600–33 167). The quantitative HIV-1 gag PCR yielded DNA concentrations in birth specimens of <1–13 708 copies/106 cells (median, 276 copies/106 cells). Five of the 62 infants tested negative by qPCR but had HIV-1 pol amplified in a median of 2 of 20 PCRs from birth DBS specimens (range, 1–20); in each case the sequence was genetically linked to a separately amplified aliquot of birth DBS or to DBS collected between 2 and 8 weeks of age; the infant's HIV-1 with 20 positive per 20 PCRs had a mutation in gag corresponding to the qPCR primer.
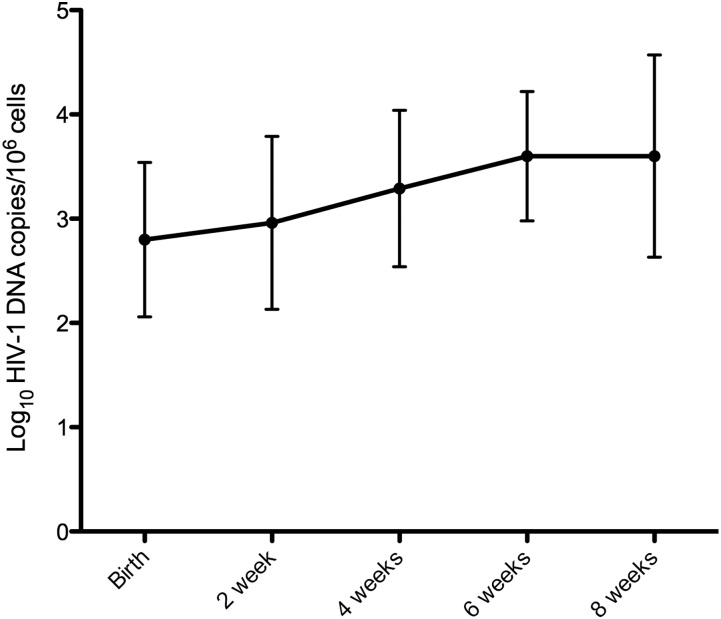
Among the infants who tested positive at birth, 27 (43%) had no additional samples, 11 (18%) had 1 additional sample between 2 and 8 weeks, 10 (16%) had 2 samples, and 14 (22%) had 3 or 4 samples. In infants with follow-up samples at or after 4 weeks (n = 29), the median HIV-1 DNA increased over time, from 450.8 copies/106 cells at birth to 6359.8 copies/106 cells at 8 weeks of life (P = .003 for Wilcoxon signed-rank test comparing these values). The HIV-1 DNA load increased by >1 log10 copies/106 cells in the majority of infants (16/29) between birth and 4–8 weeks of age (including 2 that increased between 2 and 3 log10, and 2 that increased >3 log10; Figure 1).
Figure 1.
Mean concentration (and standard deviation) of human immunodeficiency virus type 1 (HIV-1) DNA in longitudinally collected samples from the 29 HIV-1–infected infants with follow-up at or after 4 weeks, as measured by gag real-time polymerase chain reaction.
The Mozambican standard of care for maternal ART to prevent mother-to-child transmission of HIV-1 changed over the course of the study: Prior to 2006, only sdNVP was recommended for women with CD4 counts >350 cells/µL, and early in 2006 zidovudine (ZDV) was also recommended, starting as early as 32 weeks. Of the 849 women, 544 (64%) had information collected about both sdNVP and ZDV use, whereas 305 (36%) only had data on receipt of sdNVP. The quantity of HIV-1 DNA in the birth specimen did not differ according to maternal antiretroviral regimen (Table 1). Among the 21 HIV-1 DNA–positive infants with maternal ZDV use data, the 2 babies whose mothers received >7 days of ZDV had fewer positive qualitative PCR reactions than those whose mothers had <7 days of treatment (median, 3 vs 18 days; P = .04), and both groups had low HIV-1 DNA values by qPCR (median, 120 vs 510 copies/106 cells; P = .15).
Table 1.
Comparison of Mother-to-Child HIV-1 Transmission Rates and Infants’ HIV-1 DNA Concentrations at Birth Between Different Maternal Antiretroviral Treatment Conditions
| Infants’ Characteristics and Outcomes | Maternal Antiretrovirals Administered for Infant HIV-1 Prophylaxis |
|||||
|---|---|---|---|---|---|---|
| No Data Collected on ZDV Use (n = 305) |
Data Available on ZDV Use (n = 544) |
|||||
| No sdNVP | sdNVP | No ARV | sdNVP | sdNVP + ZDV | ZDV Alone | |
| Infant HIV-1 uninfected, No. | 76 | 202 | 123 | 289 | 79 | 18 |
| Infant HIV-1 infected, No. | 9 | 18 | 2 | 27 | 4 | 2 |
| Transmission rate | 11% | 8% | 2% | 9% | 5% | 10% |
| HIV-1 DNA at birth, copies/106 cells, median (IQR) | 260 (43–454) | 261 (40–1705) | 73–241a | 510 (110–2649) | 158 (113–555) | 638–1717a |
| No. (+) PCR of 20 total PCR reactions, median (IQR) | 18 (16–20) | 19.5 (14–20) | 8–18a | 18 (12–20) | 6 (3–12.5) | 20–20a |
| P valueb (comparison of HIV-1 DNA load with or without sdNVP) | .96 | .88 | ||||
| P valueb (comparison of HIV-1 DNA load with or without ZDV) | N/A | .90 | ||||
Abbreviations: ARV, antiretrovirals; HIV-1, human immunodeficiency virus type 1; IQR, interquartile range; PCR, polymerase chain reaction; sdNVP, single-dose nevirapine; ZDV, zidovudine.
a Actual values.
b Using Wilcoxon rank-sum test.
DISCUSSION
In our cohort of HIV-1–exposed sdNVP-treated Mozambican infants, one-quarter of all infants ultimately found to be HIV-1 infected before 8 weeks of age initially had a false-negative test for HIV-1 DNA at birth. The negative results were likely due to low viral load at the time of birth and testing only a small volume of blood. We suspect that if early ART can prevent the establishment of a CD4+ T-cell reservoir, that among infants, those with low copy infection may be the best target population. Whether a specific viral load threshold indicates an established long-lived reservoir that is incurable by early ART is not known, but is suggested in adults, where low Fiebig scores at ART initiation were associated with clearing or achieving low HIV-1 DNA loads and no to minimal production of HIV-1 RNA [12].
Previously, we reported that infants with lower HIV-1 DNA loads at birth established infections with persistent NVP-resistant variants more frequently than those with high DNA loads [9]. The latter most likely had already established reservoirs of wild-type viruses, suggesting that a long-lived cellular reservoir may be established in utero in a subset of infants, and we suspect that these infants may not be curable by early ART.
As our data and others have shown, identifying infants with low-copy HIV-1 infection at birth may be unreliable when testing small amounts of blood for HIV-1 DNA [12]. Simply adding DNA to commonly used assays could inhibit PCR. Detection of low concentrations of HIV-1 DNA at birth may be aided by multiple PCR assays, as performed in this study, or automated by droplet digital PCR [13]. In cases without maternal use of ART prophylaxis, testing for HIV-1 RNA should have greater sensitivity compared with testing for DNA [14].
We are limited in our analysis by the inability to determine the precise timing of infection in these infants, by missing specimens to determine the HIV-1 DNA trajectory over time in the majority of infants, and by the uncertainty of determinants that allow ART to prevent the establishment of long-lived reservoirs, or cure, HIV-1 infection.
In conclusion, a significant proportion of perinatally infected infants have low-copy HIV-1 infection at birth. These infants represent the target population most likely to achieve functional cure by immediate initiation of ART. Use of both sensitive assays and larger amounts of DNA should increase diagnosis of infant HIV-1 infections at birth.
Notes
Acknowledgments. We thank the participating women and infants; the Mozambican Ministry of Health staff; the study team (Ana Judith Blanco, Laurinda Matunha, Pablo Montoya, Eduardo Matediane, Lilia Jamisse, Stephen Gloyd, Emilia Almeida, Victoria Neves, Mfila Nhamutavira, Maria Serra, and Sandra Pereira); the laboratory staff (Joaquim Chidacaje and Jossefa Sairosse; Mozambique Ministry of Health); and Dr Joseph E. Fitzgibbon at the US National Institutes of Health (NIH) for important contributions to this study.
Financial support. This work was supported by the NIH (grant number R01 AI058723 to L. M. F.). M. A. M. was also supported in part by the NIH (STD/AIDS research training grant number T32 AI07140).
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Persaud D, Gay H, Ziemniak C, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013;369:1828–35. doi: 10.1056/NEJMoa1302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saez-Cirion A, Bacchus C, Hocqueloux L, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9:e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalish LA, Pitt J, Lew J, et al. Defining the time of fetal or perinatal acquisition of human immunodeficiency virus type 1 infection on the basis of age at first positive culture. Women and Infants Transmission Study (WITS) J Infect Dis. 1997;175:712–5. doi: 10.1093/infdis/175.3.712. [DOI] [PubMed] [Google Scholar]
- 4.Kourtis AP, Bulterys M, Nesheim SR, Lee FK. Understanding the timing of HIV transmission from mother to infant. JAMA. 2001;285:709–12. doi: 10.1001/jama.285.6.709. [DOI] [PubMed] [Google Scholar]
- 5.Walter J, Kuhn L, Semrau K, et al. Detection of low levels of human immunodeficiency virus (HIV) may be critical for early diagnosis of pediatric HIV infection by use of dried blood spots. J Clin Microbiol. 2009;47:2989–91. doi: 10.1128/JCM.02453-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steketee RW, Abrams EJ, Thea DM, et al. Early detection of perinatal human immunodeficiency virus (HIV) type 1 infection using HIV RNA amplification and detection. New York City Perinatal HIV Transmission Collaborative Study. J Infect Dis. 1997;175:707–11. doi: 10.1093/infdis/175.3.707. [DOI] [PubMed] [Google Scholar]
- 7.Coutlee F, Viscidi RP, Saint-Antoine P, Kessous A, Yolken RH. The polymerase chain reaction: a new tool for the understanding and diagnosis of HIV-1 infection at the molecular level. Mol Cell Probes. 1991;5:241–59. doi: 10.1016/0890-8508(91)90046-m. [DOI] [PubMed] [Google Scholar]
- 8.Lilian RR, Kalk E, Bhowan K, et al. Early diagnosis of in utero and intrapartum HIV infection in infants prior to 6 weeks of age. J Clin Microbiol. 2012;50:2373–7. doi: 10.1128/JCM.00431-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Micek MA, Blanco AJ, Beck IA, et al. Nevirapine resistance by timing of HIV type 1 infection in infants treated with single-dose nevirapine. Clin Infect Dis. 2010;50:1405–14. doi: 10.1086/652151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beck IA, Drennan KD, Melvin AJ, et al. Simple, sensitive, and specific detection of human immunodeficiency virus type 1 subtype B DNA in dried blood samples for diagnosis in infants in the field. J Clin Microbiol. 2001;39:29–33. doi: 10.1128/JCM.39.1.29-33.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coutlee F, He Y, Saint-Antoine P, Olivier C, Kessous A. Coamplification of HIV type 1 and beta-globin gene DNA sequences in a nonisotopic polymerase chain reaction assay to control for amplification efficiency. AIDS Res Hum Retroviruses. 1995;11:363–71. doi: 10.1089/aid.1995.11.363. [DOI] [PubMed] [Google Scholar]
- 12.Ananworanich J, Vandergeeten C, Chomchey N, et al. Early ART intervention restricts the seeding of the HIV reservoir in long-lived central memory CD4 T cells. 20th Conference on Retroviruses and Opportunistic Infections,; Atlanta, GA. 2013. [Google Scholar]
- 13.Strain MC, Lada SM, Luong T, et al. Highly precise measurement of HIV DNA by droplet digital PCR. PLoS One. 2013;8:e55943. doi: 10.1371/journal.pone.0055943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nesheim S, Palumbo P, Sullivan K, et al. Quantitative RNA testing for diagnosis of HIV-infected infants. J Acquir Immune Defic Syndr. 2003;32:192–5. doi: 10.1097/00126334-200302010-00011. [DOI] [PubMed] [Google Scholar]