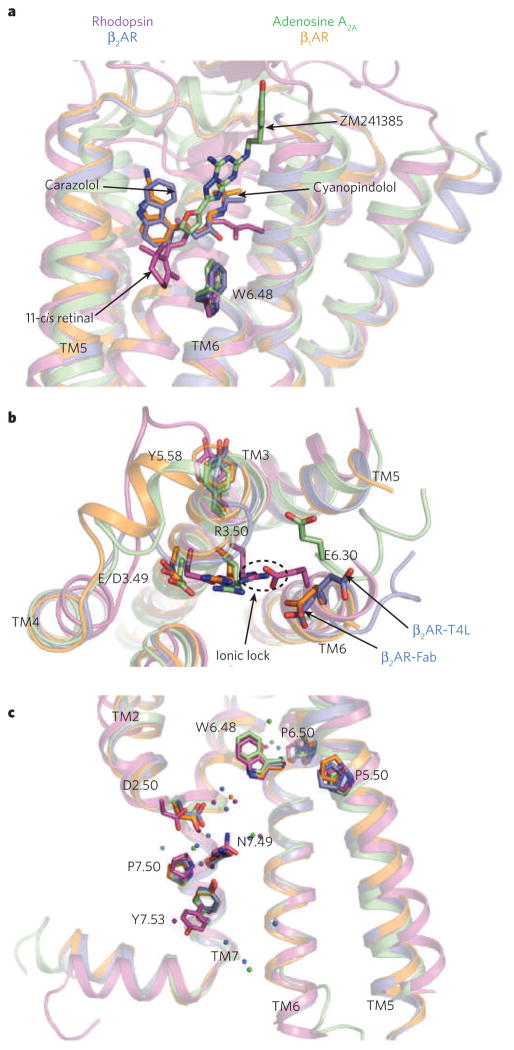
Figure 3. Comparison of conserved regions of four GPCR structures.
a, The locations of bound ligands for the four superimposed receptor structures bovine rhodopsin (purple, bound to 11-cis retinal), avian β1AR (orange, bound to cyanopindolol), human A2A adenosine receptor (green, bound to ZM241385) and human β2AR (blue, bound to carazolol) are shown. W6.48 is the key residue of the rotamer toggle switch. TM, transmembrane segment. b, The ionic-lock residues at the cytoplasmic end of TM3 (R3.50 and E/D3.49), and TM6 (E6.30) are shown for the same four structures. R3.50 engages Y5.58 on TM5, rather than E6.30 on TM6 in the opsin ‘active state’. The rotameric position of E6.30 differs for the two β2AR structures. c, The location of several highly conserved residues around a cluster of water molecules (coloured spheres) is shown. These residues may be part of a common pathway for propagating conformational changes from the ligand-binding pocket to the G-protein coupling domains. Amino acids are numbered using the Ballesteros/Weinstein numbering system29, in which the number preceding the dot refers to the transmembrane helix on which an amino acid resides. The second number designates the position relative to the most highly conserved residue among family A GPCRs, numbered 50.