Abstract
Backgroud
A biliary stricture is the most common complication after living-donor liver transplantation (LDLT). The present study was performed to examine treatment methods and outcomes after treatment for a biliary stricture after LDLT.
Methods and Results
From January 2000 to December 2010, 488 patients underwent LDLT using the right lobe with duct-to-duct anastomosis at our transplantation centre. Overall biliary strictures were detected in 160 patients (32.8%), and the majority occurred within 2 years after LDLT. Biliary strictures were related to bile leakage (P < 0.001) and the urgency of the surgery (P = 0.012) in a multivariate analysis. All biliary strictures were treated with interventional modalities including an endoscopic or a percutaneous approach. Failure of interventional treatment was demonstrated in 13 patients (8.5%), among them, four (2.6%) underwent re-transplantation and nine (5.9%) died of sepsis and biliary cirrhosis during the follow-up period. A biliary stricture was not related to the survival rate (P = 0.586).
Conclusion
The incidence of overall biliary stricture was related to bile leakage and the urgency of the surgery. All biliary strictures could be treated by interventional modalities. These approaches are effective, complementary and help to avoid the need for surgery for a biliary stricture.
Introduction
Since living-donor liver transplantation (LDLT) was first reported in 1988, LDLT using the right lobe has become a standard treatment modality for end-stage liver disease in adults.1,2 There are two methods of biliary reconstruction in LDLT using the right lobe. Roux-en-Y hepaticojejunostomy (RYHJ) is preferred for paediatric LDLT cases because the recipient bile duct is too small or because of the presence of underlying liver disease (e.g. biliary atresia). Duct-to-duct anastomosis has recently become the preferred option for adult LDLT cases because the sphincter of Oddi is preserved, physiological bilioenteric continuity is maintained, it is technically easier and endoscopic treatment is possible for biliary complications.3,4 Among the complications occurring after LDLT using the right lobe, biliary complications are the most common and may become a significant cause of morbidity and mortality in LDLT patients. The incidence of biliary leakage and stricture after LDLT using the right lobe are reportedly 4.7% to 18.2% and 8.3% to 31.7%, respectively.5–9 The development of biliary strictures is influenced by various factors including anatomical biliary variation of the graft, the reconstruction method, organ preservation, hepatic artery thrombosis, chronic rejection, and other recipient and donor characteristics.10
In the past, most biliary strictures were treated surgically. Interventional treatment has the disadvantage of requiring repeated procedures. Surgical treatment has the advantage of being definitive for biliary stricture. However, interventional treatment, including percutaneous and endoscopic approaches, has been prioritized during the past two decades because these procedures are less invasive and more convenient for the patient. Surgical treatment is performed when all interventional treatments have failed.11,12
There have been several previous reports of a biliary stricture and its risk factors in LDLT patients, but reports of long-term outcomes of patients with a biliary stricture are limited. Indeed, the numbers of patients who underwent LDLT with duct-to-duct anastomosis in these reports are also limited. In this study, we evaluated retrospectively the incidence of a biliary stricture, its contributing factors, and the treatments and outcomes of biliary stricture in patients who underwent LDLT with duct-to-duct anastomosis at a single transplant centre.
Patients and methods
Patients
From January 2000 to December 2010, a total of 488 patients underwent adult LDLT using the right lobe with duct-to-duct anastomosis at our transplant centre. The medical records of the patients were retrospectively reviewed. This study was approved by the Institutional Review Board of Seoul St. Mary's Hospital, Catholic University of Korea. Among the 488 patients, 347 (71.1%) were male and the mean age was 49.1 ± 9.3 years. The mean body mass index (BMI) was 23.8 ± 3.1 kg/m2. The mean Model for End-stage Liver Disease (MELD) score was 17.3 ± 9.7. The most common etiology of liver transplantation was hepatitis B (n = 353, 72.3%), followed by alcohol consumption (n = 46, 9.4%) and hepatitis C (n = 20, 4.1%). The number of patients on the high-urgency list (UNOS status I and IIA13) was 68 (13.9%). The mean follow-up period was 45.7 months (Table 1).
Table 1.
Clinical parameters of the study population
| Parameter | Data |
|---|---|
| No. of patients, n | 488 |
| Mean age (years)a | 49.1 ± 9.3 |
| Male, n (%) | 347 (71.1%) |
| Aetiology, n (%) | |
| Hepatitis B | 353 (72.3%) |
| Hepatitis C | 20 (4.1%) |
| Alcohol | 46 (9.4%) |
| BMI (kg/m2)a | 23.8 ± 3.1 |
| MELD scorea | 17.3 ± 9.7 |
| GRWR (%)a | 1.28 ± 0.32 |
| Fatty change in graft (>10%), n (%) | 51 (10.5%) |
| Urgency of operation, n (%)b | 68 (13.9%) |
| Operative time (min)a | 666.8 ± 117.6 |
| Blood transfusion (units)a | 13.6 ± 7.8 |
| Follow-up duration (mo)a | 45.7 ± 35.5 |
BMI, body mass index; MELD, Model for End-stage Liver Disease; GRWR, graft-to-recipient body weight ratio.
Values shown are means ± standard deviation except where otherwise stated.
Urgency of operation included UNOS I & IIA.
Biliary reconstruction method
During the recipient surgery, a hilar dissection was carefully performed to preserve an adequate blood supply to the bile duct. Care was taken to minimize dissection of the hilar region in the recipient. After hepatic vascular anastomosis, a biliary reconstruction was performed. The method of biliary reconstruction was chosen according to the number and size of graft duct openings and the anatomical variation of the biliary system. Duct-to-duct anastomosis was preferred, if possible. When the two orifices were closely located, a single duct-to-duct anastomosis was performed using ductoplasty. In contrast, when the two orifices were distantly located, a double end-to-end anastomosis was performed by anastomosing each orifice to the left and right hepatic ducts separately. The duct-to-duct anastomosis was performed using 6-0 polydiaxanone. The posterior wall is usually sutured using continuous sutures and the anterior wall using interrupted sutures under 4× magnification. Initially, T-tube placement was usually performed, but since 2005 it has only been performed in selective cases of complex anastomosis owing to biliary variations and recently it has hardly been used.
Post-operative care
All of the patients were treated with a standardized post-operative protocol. Doppler ultrasound was performed every other day during the first week, and weekly during the first month. On the 7 and 20th post-operative days, follow-up liver computed tomography (CT) scans were performed for evaluation of vascular status and liver regeneration. On the 20th post-operative day, magnetic resonance cholangio pancreatography (MRCP) was performed to evaluate the biliary status. CT scans were performed annually thereafter or in the presence of abnormal clinical conditions. MRCP was performed when biliary complications were suspected. A biliary stricture was diagnosed when stenosis of the bile duct and dilatation of the intrahepatic duct proximal to the stricture were observed on abdominal CT or MRCP and when liver biochemical parameters, such as serum bilirubin, alkaline phosphatase and γ-glutamyl transferase, were abnormal. The outcomes were divided into success and failure. The treatment success group included the patients who reached stent-free status and the patients who had a stent with normal liver biochemical parameters.
Management of biliary stricture
Whether endoscopic retrograde biliary drainage (ERBD) or percutaneous transhepatic biliary drainage (PTBD) is chosen as the first treatment after diagnosis of a biliary stricture depends on the status of the biliary stricture and the patient's condition. In general, ERBD is recommended as the first approach. In this study, patients in whom endoscopic treatment failed were rescued with PTBD. If primary or rescue PTBD was successfully performed with internal drainage, the PTBD catheter was changed to an ERBD stent using the rendezvous technique. After successful insertion of the stent, follow-up ERBD was performed within 6 months. During the follow-up ERBD, the previous stent was removed or changed to a new stent. Patients in whom ERBD and PTBD failed underwent magnetic compression anastomosis.
ERBD
ERBD was performed using a video duodenoscope (Ed-450XT5; Fujinon, Saitama City, Saitama, Japan) with the patient in a prone position. The bile duct was cannulated and a fluoroscopic image was obtained using a contrast agent to evaluate the type and site of the stricture. An attempt to advance the guidewire past the stricture site was made, and when difficult, a smaller-gauge guidewire was used. After guidewire passage, a bougination catheter or balloon catheter was used to attempt dilatation of the stricture site. Next, we tried to insert as many stents (7–11.5 F in diameter, 10–16 cm in length; Wilson-Cook Medical, Achenmuhle, Germany) as possible, with the largest diameters possible. A minor sphincterotomy was performed in all patients in whom an ERBD stent was inserted.
PTBD
Intrahepatic duct dilatation was identified using ultrasonography with the patient in a supine position. Under fluoroscopic guidance, a 21-G Chiba needle was used to puncture the dilated intrahepatic duct. After insertion of a 0.018-inch hairwire, a yellow sheath followed by a 0.035-inch guidewire was introduced. A tubogram was performed to identify the stricture, and an 8-F pigtail catheter was inserted so that the tip was placed in the common bile duct (CBD) or duodenum.
Rendezvous method
When a PTBD catheter was inserted past the stricture site and internal drainage was being performed, the rendezvous method was used to change the PTBD catheter to an ERBD catheter. First, the PTBD catheter was used to perform a cholangiogram. A guidewire (0.035-inch Jagwire; Boston Scientific, Natick, MA, USA) was inserted through the PTBD catheter so that the tip was placed in the duodenum, and the PTBD catheter was removed. Using a duodenoscope, the guidewire passing through the ampulla down to the duodenum was identified. A bottle-top metal-tip ERBD catheter (MTW Endoskopie, Wesel, Germany) was passed along the guidewire through the stricture site, and the guidewire was removed. The subsequent procedure was the same as that for ERBD explained above.
Magnetic compression method
After insertion of a PTBD catheter, the tract was dilated to 18 F. Using ERBD, a self-expendable metal stent was inserted into the CBD. A magnet was placed in the CBD, distal to the stricture site. Another magnet was placed proximal to the stricture site through a percutaneous tract. As the magnets became approximated over time, simple abdominal films were taken to check the location of the magnets every 2 weeks. If a new tract was successfully formed, the magnets passed naturally through the body. The internal drainage catheter was inserted through the recanalized anastomosis site.
Statistical analysis
Continuous variables are reported as means ± standard deviation. To evaluate the risk factors for a biliary stricture, univariate analysis of risk factors was performed using a chi-square test for categorical variables and Student's t-test for continuous variables. Variables that appeared to be significantly associated with biliary stricture (P < 0.05) were entered into a multivariate analysis using binary logistic regression. Overall survival was calculated using the Kaplan–Meier method and evaluated with the log-rank test. Statistical analysis was performed using SPSS (Chicago, IL, USA) 18.0 for Windows. A P-value < 0.05 was considered to indicate statistical significance.
Results
Anatomical biliary variation and the method for biliary reconstruction
According to Couinaud's classification of biliary anatomy, the grafts of 342 patients (70.1%) were type 1 (no variation), those of 33 patients (6.8%) were type 2 (trifurcation of the bile duct), those of 57 patients (11.7%) were type 3 (right posterior duct drains into the left hepatic duct) and those of 56 patients (11.4%) were type 4 (right posterior duct drains into the common hepatic duct) (Fig. 1). A total of 146 patients (29.9%) had multiple bile duct openings in the graft, including 134 patients who had two openings, 10 patients with three openings and two patients with four openings.
Figure 1.
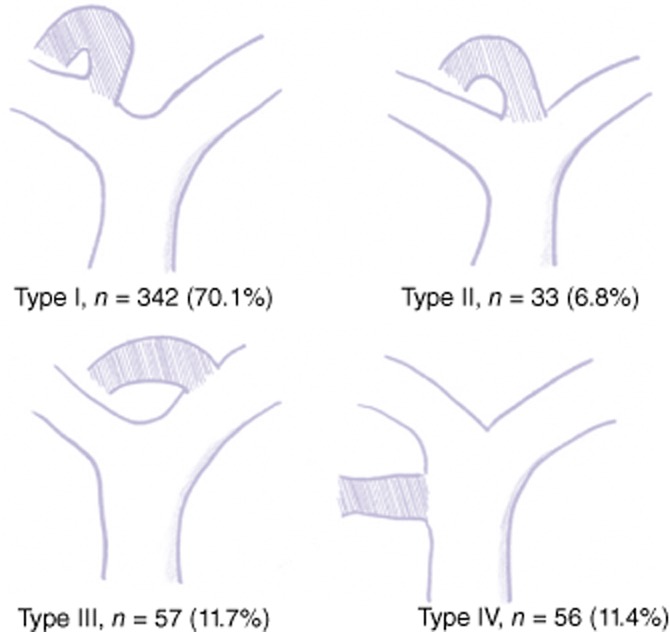
Anatomical variation of the right hepatic duct
In all 342 patients with a single-duct opening, single duct-to-duct anastomosis was performed. In 96 (71.6%) of the 134 patients with a double-duct opening, a ductoplasty was performed, and single anastomosis to the recipient CBD was performed. In the remaining 38 patients (28.4%), double duct-to-duct anastomosis using the left and right hepatic ducts separately (or to the common hepatic duct or cystic duct) was performed. In five of the 10 patients with three right bile duct lumens, ductoplasty double duct-to-duct anastomosis was performed. In the remaining five patients, a ductoplasty (three lumens) duct-to-duct anastomosis was performed. In both patients with four right bile duct lumens, double-plasty double duct-to-duct anastomosis was performed.
Overall incidence of biliary stricture and risk factors
Biliary strictures were observed in 160 patients (32.8%). The majority (96.3%) of biliary strictures occurred within 2 years after liver transplantation. The cumulative incidences of a biliary stricture in 1, 2, and 5 years were 26.8%, 31.4% and 32.6% respectively. According to the univariate analysis, the urgency of the surgery (UNOS status I and IIA) (P = 0.032), multiple openings of the graft bile duct (P = 0.033), the use of ductoplasty (P = 0.026) and a history of bile leakage (P < 0.001) were significantly associated with biliary stricture (Table 2). These four factors were entered into a multiple logistic regression analysis. Urgency of the surgery (UNOS status I and IIA) [odds ratio (OR), 2.003 (1.164–3.439), P = 0.012] and a history of bile leakage (OR, 4.566 (2.758–7.558), P < 0.001) were found to be significant factors associated with a biliary stricture (Table 3). Patient age, donor age, BMI, Child score, MELD score, the graft-to-recipient body weight ratio and the severity of fatty change in the graft were not significantly different between the two groups of patients. Biliary variation, bile duct size, use of a T-tube, operation time and the blood transfusion volume were not significant factors for a biliary stricture. Also hepatic artery thrombosis history was not a significant factor for a biliary stricture. When there were two lumens (n = 134), the frequency of a biliary stricture in the ductoplasty group (n = 96, 71.6%) and the double duct-to-duct group (n = 38, 28.4%) was 41.7% and 34.2% respectively, with no significant difference (P = 0.426).
Table 2.
Univariate analysis of factors associated with a biliary stricture
| Factor | Stricture (n = 160) | Non-stricture (n = 328) | P-value |
|---|---|---|---|
| Patient age (years)a | 50.0 ± 8.9 | 48.7 ± 9.3 | 0.134 |
| Patient; male, n (%) | 121 (75.6) | 226 (68.9) | 0.124 |
| Donor age (years)a | 34.4 ± 10.9 | 32.7 ± 11.2 | 0.122 |
| Donor; male, n (%) | 96 (60.0) | 220 (67.1) | 0.125 |
| BMI (kg/m2)a | 23.6 ± 2.7 | 23.8 ± 3.2 | 0.494 |
| Child scorea | 9.4 ± 2.5 | 9.4 ± 2.4 | 0.974 |
| MELD score (>25), n (%) | 40 (25.0) | 71 (21.6) | 0.407 |
| Urgency of the surgery, n (%) | 30 (18.8) | 38 (11.6) | 0.032 |
| GRWR (<1%), n (%) | 29 (18.1) | 53 (16.2) | 0.585 |
| Fatty change (>10%), n (%) | 20 (12.5) | 31 (9.5) | 0.301 |
| Biliary variation, n (%) | 51 (31.9) | 89 (27.1) | 0.277 |
| Multiple duct opening, n (%) | 58 (36.2) | 88 (26.8) | 0.033 |
| Bile duct size (mm)a | 4.37 ± 1.59 | 4.70 ± 1.80 | 0.255 |
| Ductoplasty, n (%) | 45 (28.1) | 63 (19.2) | 0.026 |
| Double anastomosis, n (%) | 13 (8.1) | 25 (7.6) | 0.846 |
| T-tube insertion, n (%) | 52 (32.5) | 134 (40.9) | 0.074 |
| Operation time (min)a | 628.8 ± 98.4 | 643.3 ± 125.9 | 0.201 |
| Blood transfusion (units)a | 13.7 ± 7.1 | 13.5 ± 8.1 | 0.855 |
| Bile leakage, n (%) | 53 (33.1) | 32 (9.8) | <0.001 |
| Hepatic artery thrombosis, n (%) | 4 (2.5%) | 8 (2.4%) | 0.967 |
Urgency of the surgery included UNOS I & IIA. BMI, body mass index; MELD, Model for End-stage Liver Disease; GRWR, graft-to-recipient body weight ratio.
Values shown are means ± standard deviation except where otherwise stated.
Table 3.
Multivariate analysis of factors associated with a biliary stricture
| Factor | Odds ratio (95% confidence interval) | P-value |
|---|---|---|
| Urgency of the surgerya | 2.003 (1.164–3.439) | 0.012 |
| Multiple duct openings | 0.792 (0.384–1.634) | 0.528 |
| Bile leakage | 4.566 (2.758–7.558) | <0.001 |
| Ductoplasty | 1.625 (0.736–3.588) | 0.229 |
Urgency of the surgery included UNOS I & IIA.
Treatment and clinical outcome of biliary stricture
The median time of first interventional treatment from LDLT was 6 months, the mean number of biliary interventions was 3.2 (range, 1 to 11) and the median interval time between interventions was 4 months (range, 1 to 33).
Excluding the seven patients lost to follow-up, of the 153 patients with a biliary stricture, 65 initially underwent ERBD (42.5%) and 88 initially underwent PTBD (57.5%) (Fig. 2). Of the 65 patients who underwent ERBD, 53 (81.5%) had a stent inserted through the ERBD, 12 (18.5%) of whom could not undergo endoscopic treatment because of difficulty in accessing the papilla of Vater and passing a guidewire through the tight anastomotic stricture. Of the 12 patients who could not undergo endoscopic treatment, eight underwent PTBD, three underwent re-transplantation and one died. Of the eight patients who underwent PTBD, five were treated successfully with PTBD, ERBD and the magnetic compression method, and three died. Of the patients treated with ERBD, interventional treatment failed in seven patients (10.8%). Of these seven patients, three underwent re-transplantation and four (6.2%) died.
Figure 2.
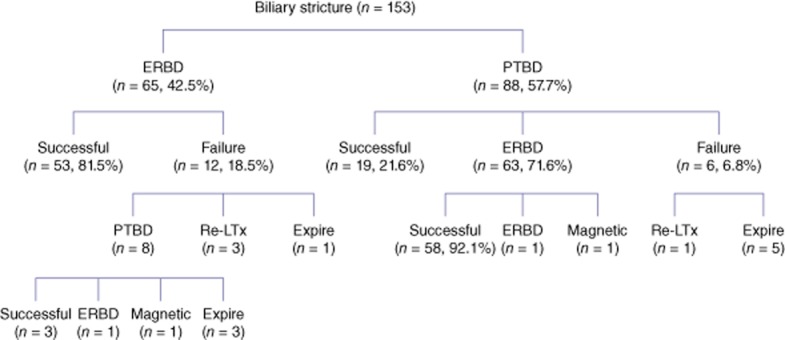
Treatment and clinical outcomes in 153 patients with a biliary stricture after living-donor liver transplantation. ERBD, endoscopic retrograde biliary drainage; PTBD, percutaneous transhepatic biliary drainage; Re-LTx, re-transplantation; Magnetic, magnetic compression method
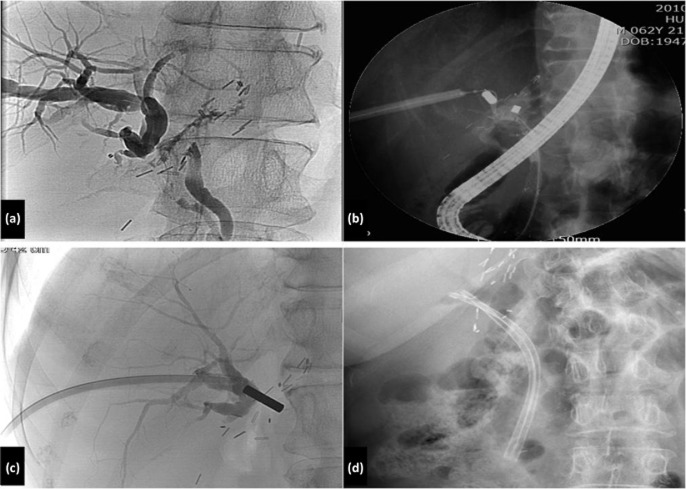
A total of 88 patients (57.5%) with a biliary stricture were initially managed with PTBD. Sixty-three (71.6%) of them were managed using the rendezvous method, 19 (21.6%) were treated solely with PTBD and treatment failed in six (6.8%). Of these six patients, one underwent re-transplantation and five (5.7%) died. Of the 63 patients who underwent the rendezvous method, 58 (92.1%) were successfully treated with this procedure alone. The remaining patients were treated with other procedures such as PTBD and the magnetic compression method. Three patients in whom PTBD and endoscopic retrograde cholangiopancreatography (ERCP) had both failed as a result of complete obstruction at the anastomosis site were all successfully re-canalized using the magnetic compression method (Fig. 3).
Figure 3.
Magnetic compression method in patients with complete biliary obstruction after living-donor liver transplantation. (a) Complete obstruction of a relatively long bile duct in cholangiography. (b) A magnet combined with a polypectomy snare was delivered via endoscopic retrograde cholangiopancreatography (ERCP) through the common bile duct. Another magnet was fixed to an alligator forceps and moved toward the anastomosis site through a percutaneous tract. (c) The magnets were approximated. (d) Two endoscopic retrograde biliary drainage (ERBD) catheters were inserted through the re-canalized tract
All patients with a biliary stricture could be treated by interventional modalities such as endoscopic, percutaneous or both. Interventional treatment failed in 13 patients (8.5%), of whom four survived with re-transplantation and nine (5.9%) died. The cause of death was biliary sepsis owing to recurrent cholangitis and a liver abscess as well as graft failure because of biliary cirrhosis. Treatment failure occurred mostly in the early period of our transplantation experience, and since 2007, there were no deaths as a result of a biliary stricture. The patients with biliary strictures had 1-, 3-, and 5-year overall survival rates of 93.7%, 85.0% and 82.4%, respectively, whereas the patients without biliary strictures had overall survival rates of 91.2%, 87.7% and 86.3%, respectively. There were no significant differences in overall survival rates between the two groups (P = 0.586) (Fig. 4).
Figure 4.
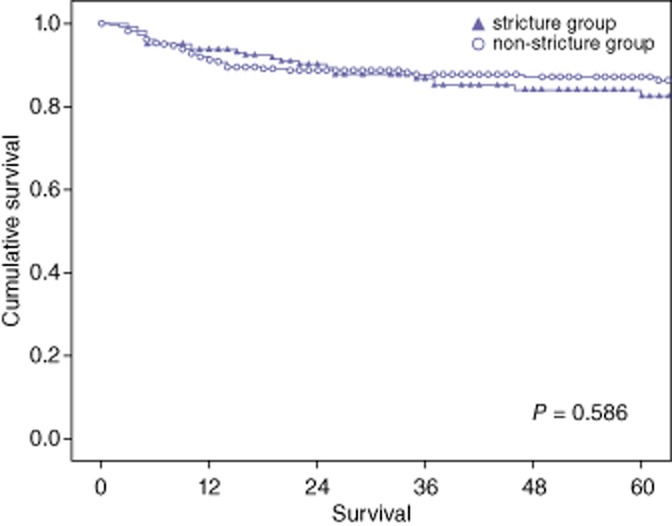
Overall survival in patients with or without a biliary stricture
Discussion
Biliary complications after LDLT are considered to be the technical Achilles' heel of liver transplantation because of their high frequency, the need for long-term and repeated treatment, and the potential detrimental effects on graft and patient survival. Although there have been recent improvements in donor selection, organ preservation, surgical techniques and immunosuppressive management, biliary strictures are still the most common complication after liver transplantation.5,6 Compared with deceased-donor liver transplantation (DDLT), biliary complications occur in LDLT more often than in DDLT.14–16 This is considered to be related to the blood supply of the anastomosis and the multiple small ducts of the grafts. Because the diameter of the bile duct anastomosis is larger, the ischaemic time is longer and the liver quality is poorer in DDLT, multiple intrahepatic bile duct strictures are more common than anastomotic strictures. In contrast, anastomotic strictures owing to anastomosis of a small bile duct are much more common in LDLT. Because of these well-known advantages, we prefer duct-to-duct anastomosis when the recipient duct is not diseased. Akamatsu et al.16 reported that duct-to-duct anastomosis was the procedure of choice in most institutions (73%) and in most patients (92%) in DDLT populations, whereas many institutes still adopted bilioenterostomy for a considerable proportion of LDLT patients (31%).
The incidences of biliary leakage and stricture after LDLT using the right lobe are reportedly 4.7% to 18.2% and 8.3% to 31.7%, respectively,5–9 with considerable variation between reports. This is thought to result from the differing definitions of a biliary stricture. In the present study, the biliary stricture rate was 32.8%. This rate is somewhat higher than that in other studies, probably because we used a relatively wider range of diagnosis. The development of biliary strictures has been reported to be related to many conditions, including anatomical biliary variations of the graft, the method of bile duct reconstruction, prolonged cold ischaemic time, hepatic artery thrombosis, blood group incompatibility, cytomegalovirus infection, the use of reduced-size grafts, the use of the University of Wisconsin solution, chronic rejection and other recipient and donor characteristics.8–10 Biliary leakage is reportedly one of the most important factors in the development of biliary strictures.10 In the present study, a history of bile leakage was found to be significantly associated with a biliary stricture in multivariate analysis. The urgency of transplantation was also significantly associated with a biliary stricture in the present study. These findings indicate that emergency surgery is a high-risk factor of a biliary stricture because of an insufficient understanding of the biliary anatomy of LDLT donors and the difficulties in selection of an optimal donor. According to Liu et al.,17 the high-MELD-score group (>35) had significantly more biliary complications (50% versus 13%, P = 0.044), and although statistically non-significant, the high-urgency transplantation group had a tendency to suffer more biliary complications (36% versus 11%, P = 0.075). An accurate pre-operative evaluation of the biliary anatomy in LDLT donors and establishment of an appropriate surgical design can minimize biliary complications in recipients. In the present hospital, pre-operative contrast-enhanced MRC and intra-operative cholangiography in elective LDLT are routinely performed.
Surgical and interventional treatments are available for biliary strictures. Although surgical treatment has the advantage of the possibility of a definite cure, because it is a second operation, identification of the anastomosis and re-anastomosis can be technically difficult, and surgical complications may occur. On the other hand, interventional treatment is less invasive and more comfortable for the patient. Because of this, surgical treatment is reserved for patients in whom all modalities of interventional treatment have failed. The two main options of interventional treatment are ERBD and PTBD. ERBD has several advantages compared with PTBD. ERBD involves fewer complications during and after the procedure, but cannulation can be relatively difficult. During transplantation surgery, the site of bile duct anastomosis is generally linear. However, because LDLT is a partial transplantation with rapid regeneration of the graft liver, the bile duct anastomosis site tends to angulate to the right, making cannulation past the anastomosis site difficult. In addition, if the cannulation is not performed past the stricture site, the contrast agent can cause cholangitis. The PTBD catheter reduces the patient's quality of life and induces catheter-related complications such as leakage, pain, infection and accidental removal of the catheter. Occasionally, it can even cause vessel injury, resulting in serious complications such as vascular occlusion. However, cannulation past the anastomosis site is relatively easy to perform. Although ERBD reportedly has a success rate and patency similar to those of PTBD,18,19 in this study, the cannulation failure rate was higher in ERBD than in PTBD (18.5% versus 6.8%); the treatment failure rate was also higher in ERBD (10.8% versus 6.8%). Recently, the rendezvous method, which utilizes both of these procedures, has been used frequently. The rendezvous procedure combines the endoscopic technique with the percutaneous transhepatic approach to increase the success rate of biliary tract cannulation in cases in which previous endoscopic attempts have failed. In earlier periods, the rendezvous method showed good results when used on patients with choledocholithiasis that was difficult to treat with ERCP.20,21 In our centre, based on this experience, the rendezvous method was used to change the primary or rescue PTBD to an ERBD catheter in patients with a biliary stricture after LDLT. The results show that this method has a high success rate. The rendezvous procedure was performed on 41% of all patients, and all patients were treated successfully. The rendezvous procedure has recently become the treatment of choice for biliary stricture in our centre.
Patients in whom ERC and PTBD have failed to establish recanalization must maintain an external PTBD catheter to support life. In 1998, Yamanouchi et al.22 introduced magnetic compression anastomosis. In this procedure, transmural compression of two magnets causes gradual ischaemic necrosis, creating a new anastomosis. Later, Takao et al.23 and Mimuro et al.24 successfully applied this method to patients with a benign CBD obstruction. In our centre, this method is used on patients with a biliary stricture after LDLT in whom both PTBD and ERCP have failed. Thus far, three patients have undergone magnetic compression anastomosis, and the biliary anastomotic stricture was recanalized successfully in all three.
Because the main cause of death when all interventional treatments have failed is sepsis, prevention of infection is important. In addition, when biliary cirrhosis causes graft dysfunction, re-transplantation should immediately be considered. Most deaths caused by a biliary stricture occurred in earlier periods, when our experience was relatively lacking. There have been no deaths as a result of a biliary stricture since 2007. One reason for this improvement is that based on our accumulated experience, we are now actively searching for biliary complications according to a standardized protocol and aggressively treating any discovered complications in the early stages. Second, through interdepartmental consultations between LT-related teams, procedures such as ERCP and PTBD have improved over time.
The interventional approach is mandatory in the management of a biliary stricture. However, the long-term outcomes of a biliary stricture after an interventional approach have not been clearly defined, especially in patients who have undergone LDLT with duct-to-duct anastomosis. This study showed that 104 (65.0%) patients achieved a stent-free status with normal liver function and that 36 (22.5%) patients were under treatment during the mean follow-up period of 45.7 months. In addition, there were no significant differences in overall survival rates between the biliary stricture group and the non-biliary stricture group. This shows that interventional modalities such as endoscopic and/or percutaneous approaches are effective and have an acceptable long-term outcome.
In conclusion, a biliary stricture was related to bile leakage and the urgency of the surgery. Because the majority of biliary strictures occurred within 2 years after LDLT, careful follow-up is required for patients with risk factors, especially within the first 2 years. All biliary strictures could be treated by interventional modalities, such as endoscopic and/or percutaneous approaches. These approaches are effective and complementary treatment modalities that help to avoid the need for surgery.
Conflicts of interest
Gun Hyung Na, Ho Joong Choi, Jae Hyun Han, Tae Ho Hong, Young Kyoung You and Dong Goo Kim have no conflict of interest or financial ties to disclose.
References
- 1.Hashikura Y, Makuuchi M, Kawasaki S, Matsunami H, Ikegami T, Nakazawa Y, et al. Successful living–related partial liver transplantation to an adult patient. Lancet. 1994;343:1233–1234. doi: 10.1016/s0140-6736(94)92450-3. [DOI] [PubMed] [Google Scholar]
- 2.Liu CL, Fan ST, Lo CM, Wi W, Chan SC, Yong BH, et al. Operative outcomes of adult-to-adult right lobe live donor liver transplantation: a comparative study with cadaveric whole-graft liver transplantation in a single center. Ann Surg. 2006;243:404–410. doi: 10.1097/01.sla.0000201544.36473.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan ST, Lo CM, Liu CL, Tso WK, Wong J. Biliary reconstruction and complications of right lobe live donor liver transplantation. Ann Surg. 2002;236:676–683. doi: 10.1097/00000658-200211000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishiko T, Egawa H, Kasahara M, Nakamura T, Oike F, Kaihara S, et al. Duct-to-duct biliary reconstruction in living donor liver transplantation utilizing right lobe graft. Ann Surg. 2002;236:235–240. doi: 10.1097/00000658-200208000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Testa G, Malagò M, Broelseh CE. Complications of biliary tract in liver transplantation. World J Surg. 2001;25:1296–1299. doi: 10.1007/s00268-001-0113-5. [DOI] [PubMed] [Google Scholar]
- 6.Wojcicki M, Milkiewicz P, Silva M. Biliary tract complications after liver transplantation: a review. Dig Surg. 2008;25:245–247. doi: 10.1159/000144653. [DOI] [PubMed] [Google Scholar]
- 7.Kasahara M, Egawa H, Takada Y, Oike F, Sakamoto S, Kiuchi T, et al. Biliary reconstruction in right lobe living-donor liver transplantation: comparison of different techniques in 321 recipients. Ann Surg. 2006;243:559–566. doi: 10.1097/01.sla.0000206419.65678.2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yazumi S, Chiba T. Biliary complications after a right-lobe living donor liver transplantation. J Gastroenterol. 2005;40:861–865. doi: 10.1007/s00535-005-1698-5. [DOI] [PubMed] [Google Scholar]
- 9.Hwang S, Lee SG, Sung KB, Park KH, Ahn CS, Lee YJ, et al. Long-term incidence, risk factors, and management of biliary complications after adult living donor liver transplantation. Liver Transpl. 2006;12:831–838. doi: 10.1002/lt.20693. [DOI] [PubMed] [Google Scholar]
- 10.Verdonk RC, Buis CI, Porte RJ, van der Jagt EJ, Limburg AJ, van den Berg AP, et al. Anastomotic biliary strictures after liver transplantation: causes and consequences. Liver Transpl. 2006;12:726–735. doi: 10.1002/lt.20714. [DOI] [PubMed] [Google Scholar]
- 11.Lopez RR, Benner KG, Ivancev K, Keeffe EB, Deveney CW, Pinson CW. Management of biliary complications after liver transplantation. Am J Surg. 1992;163:519–524. doi: 10.1016/0002-9610(92)90401-c. [DOI] [PubMed] [Google Scholar]
- 12.Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation: past, present and preventive strategies. Liver Transpl. 2008;14:759–769. doi: 10.1002/lt.21509. [DOI] [PubMed] [Google Scholar]
- 13.United Network for Organ Sharing. 2012. Allocation of Livers. Available at http://optn.transplant.hrsa.gov/PoliciesandBylaws2/policies/pdfs/policy_8.pdf(last accessed June 2013)
- 14.Alonso EM, Piper JB, Echols G, Thistlethwaite JR, Whitington PF. Allograft rejection in pediatric recipients of living related liver transplants. Hepatology. 1996;23:40–43. doi: 10.1002/hep.510230106. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka K, Uemoto S, Tokunnaga Y, Fujita S, Sano K, Nishizawa T, et al. Surgical techniques and innovations in living related liver transplantation. Ann Surg. 1993;217:82–91. doi: 10.1097/00000658-199301000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akamatsu N, Sugawara Y, Hashimoto D. Biliary reconstruction, its complications and management of biliary complications after adult liver transplantation: a systematic review of the incidence, risk factors and outcome. Transpl Int. 2011;24:379–392. doi: 10.1111/j.1432-2277.2010.01202.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu CL, Lo CM, Chan SC, Fan ST. Safety of duct-to-duct biliary reconstruction in right-lobe live-donor liver transplantation without biliary drainage. Transplantation. 2004;77:726–732. doi: 10.1097/01.tp.0000116604.89083.2f. [DOI] [PubMed] [Google Scholar]
- 18.Lee SH, Ryu JK, Woo SM, Park JK, Yoo JW, Kim YT, et al. Optimal interventional treatment and long-term outcomes for biliary stricture after liver transplantation. Clin Transplant. 2008;22:484–493. doi: 10.1111/j.1399-0012.2008.00813.x. [DOI] [PubMed] [Google Scholar]
- 19.Londoño MC, Balderramo D, Cárdenas A. Management of biliary complications after orthotopic liver transplantation: the role of endoscopy. World J Gastroenterol. 2008;14:493–497. doi: 10.3748/wjg.14.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scapa E, Peer A, Witz E, Eshchar J. ‘Rendez-vous’ procedure (RVP) for obstructive jaundice. Surg Laparosc Endosc. 1994;4:82–85. [PubMed] [Google Scholar]
- 21.Ponchon T, Valette PJ, Bory R, Bret PM, Bretagnolle M, Chavaillon A. Evaluation of a combined percutaneous-endoscopic procedure for the treatment of choledocholithiasis and benign papillary stenosis. Endoscopy. 1987;19:164–166. doi: 10.1055/s-2007-1018270. [DOI] [PubMed] [Google Scholar]
- 22.Yamanouchi E, Kawaguchi H, Endo I. A new interventional method: magnetic compression anastomosis with rare-earth magnets. Cardiovasc Intervent Radiol. 1998;21(Suppl. 1):S155. [Google Scholar]
- 23.Takao S, Matsuo Y, Shinchi H, Nakajima S, Aikou T, Iseji T, et al. Magnetic compression anastomosis for benign obstruction of the common bile duct. Endoscopy. 2001;33:988–990. doi: 10.1055/s-2001-17923. [DOI] [PubMed] [Google Scholar]
- 24.Mimuro A, Tsuchida A, Yamanouchi E, Itoi T, Ozawa T, Ikeda T, et al. A novel technique of magnetic compression anastomosis for severe biliary stenosis. Gastrointest Endosc. 2003;58:283–287. doi: 10.1067/mge.2003.354. [DOI] [PubMed] [Google Scholar]