Abstract
Background
Peri-operative chemotherapy is recommended for the management of colorectal liver metastases (CRLM). The aim of this study was to examine the impact of peri-operative bevacizumab on survival in patients with resected CRLM.
Methods
A multicentre retrospective cohort of patients with resected CRLM was analysed from the LiverMetSurvey Registry. Patients who received peri-operative FOLFOX (group A) were compared with those who received peri-operative FOLFOX and bevacizumab (group B).
Results
In total, 501 patients were compared (A, n = 384; B, n = 117). Group A was older (68.3 versus 62.5 years, P < 0.01), had more rectal cancers (30.7 versus 18.8%, P < 0.01) and higher carcinoembryonic antigen (CEA) levels at diagnosis (17.0 versus 9.7 ng/ml, P = 0.043). No difference was observed regarding primary tumour stage, synchronicity and the number or size of metastases. Post-operative infections were more frequent in group B (4.7% versus 12.8%, P < 0.01). Peri-operative bevacizumab had no effect on 3-year overall survival (OS) (76.4% versus 79.8%, P = 0.334), or disease-free survival (DFS) (7.4% versus 7.9%, P = 0.082). DFS was negatively associated with primary tumour node positivity (P = 0.011) and synchronicity (P = 0.041).
Conclusions
The addition of bevacizumab to standard peri-operative chemotherapy does not appear to be associated with improved OS or DFS in patients with resected CRLM.
Introduction
Colorectal cancer (CRC) is the second cause of cancer-related death in western countries.1 Colorectal liver metastases (CRLM) develop in nearly half of patients with CRC, and approximately 80–90% of these will initially be unresectable.2,3 Complete resection of hepatic metastases is curative in selected patients,4 and 5-year survival rates vary from 25% to 40% after a hepatectomy.5–7 However, up to 60% of patients develop recurrent metastases within the first 2 years after a hepatic resection.8 This suggests possible unrecognized metastatic microfoci at the time of liver metastasectomy, and emphasizes the role of systemic chemotherapy in the management of CRLM. Improved 5-and 10-year survival rates up to 58% and 36% are obtainable, respectively, when a multimodality strategy of chemotherapy and surgery is used.9–12 The addition of bevacizumab, a monoclonal antibody directed against the vascular endothelial growth factor (VEGF), to first and second line pre-operative chemotherapy for metastatic CRC was shown to increase resectability of liver metastases and statistically improve overall survival (OS) and disease-free survival (DFS) in all patients with stage IV disease.13,14
The phase III clinical trial by the European Organization for Research and Treatment of Cancer (EORTC) demonstrated that peri-operative FOLFOX4 significantly increases DFS at 3 years in patients with resectable CRLM.15 Chemotherapy in conjunction with a hepatic resection has since then become the standard treatment of CRLM. The current recommended regimens include FOLFOX, XELOX, or FOLFIRI in conjunction with a targeted biological agent such as bevacizumab in the pre-operative setting, and cytotoxic agents alone in the post-operative setting.16–18 The efficacy of adjuvant bevacizumab has not been demonstrated for stage II and III CRC.19,20 As a logical extension, bevacizumab is not recommended by expert panels to be included in the adjuvant treatment of CRLM, unless a benefit was shown in the neoadjuvant setting.
There is currently a paucity of data examining the addition of bevacizumab to modern peri-operative chemotherapy in the context of resectable CRLM. Thus, the objective of this work was to report a retrospective analysis of a large multicentre database on the impact of bevacizumab added to peri-operative FOLFOX for patients with resected CRLM, focusing on OS and DFS.
Patients and methods
A retrospective review of a multicentre cohort of patients resected for CRLM between 2002 and 2012 was conducted. Data for this study were obtained from the LiverMetSurvey International Registry. The LiverMetSurvey is a prospective international online database of patients with resected metastatic CRC.21 The database includes data voluntarily registered by more than 250 centres across 52 countries. All clinical treatment decisions pertaining to patients within the database were made by individual clinicians and were not standardized for this study. Demographic, tumour-related, peri-operative treatment and survival data, as well as duration of chemotherapy regimens were collected from the database and analysed.
Patients who had undergone a liver resection for synchronous or metachronous CRLM, and who were treated with peri-operative FOLFOX, with or without bevacizumab, were included. Patients under fluoropyrimidine-based, irinotecan-based regimens or XELOX were excluded. Eligible patients were separated into two groups for comparison: patients treated with peri-operative FOLFOX (group A), and patients treated with peri-operative FOLFOX plus pre-operative bevacizumab or peri-operative bevacizumab (group B). The decision to utilize bevacizumab was made by individual clinicians and was not standardized or recorded. Data pertaining to the original resectability status of individual patients were available and were included in this study.
OS was defined as the time period from liver metastasectomy to the date of death or to the date of the last follow-up. DFS was defined as the time period from liver resection to the date of proven recurrence or the date of death. Synchronous CRLM was defined based on the existing LiverMetSurvey definition of 6 months. A major liver resection was defined as the resection of three or more liver segments. The term ‘peri-operative’ was used to refer to chemotherapy regimens administered to patients prior to and after liver surgery. A response was reported with World Health Organization criteria in conjunction with clinical evaluation as determined by LiverMetSurvey.
Group A was compared with group B based on several variables: patient demographics, primary tumour characteristics and stage, liver metastasis characteristics, liver surgery parameters, post-operative complications, chemotherapeutic regiments, disease recurrence and survival. For survival analyses, the cohort was restricted to patients who had a minimum follow-up of 12 months after a hepatectomy.
Pearson's χ2 test and the Mann–Whitney U-test were used where appropriate. OS and DFS for individual groups were estimated using the Kaplan–Meier method and then compared using the log-rank test. A Cox's proportional hazard multivariate regression model was constructed. Univariate analysis was first conducted and associated factors with P ≤ 0.10 were included in the multivariate analysis. Factors with P ≤ 0.05 in the multivariate analysis were considered to be independent predictors of OS or DFS. Given the study objective, the addition of peri-operative bevacizumab was included in the multivariate analysis, irrespective of its statistical association in the univariate analysis. All statistical calculations were performed using SPSS Statistics (version 20, SPSS Inc., Chicago, IL, USA).
Results
Overview
A total of 501 patients from the registry over a span of 10 years (2002–2012) met the inclusion criteria: 384 patients in group A, 117 patients in group B (66 patients received peri-operative bevacizumab and 51 patients received pre-operative bevacizumab). The median follow-up time for all patients was 22 months (range: 2–203). Patients in group A were followed for a median of 25 months, compared with 14 months for patients in group B. After restricting for a minimum of 12 months of follow-up for survival analysis, the overall median follow-up time was 32 months (35 versus 24 months). Clinical characteristics for both groups are listed in Table 1. Both groups were similar except for age, type of primary cancer and carcinoembryonic antigen (CEA) levels at diagnosis. In group B, the number of patients treated with bevacizumab was comparable between the first half and the second half of the study (48% versus 53%). Patients with synchronous liver disease underwent a resection of their primary tumour, received chemotherapy then a hepatectomy for CRLM. In the context of a simultaneous colorectal and hepatic resection (50 patients; 13.0% versus 10 patients; 8.5%, P = 0.343), patients underwent pre-operative chemotherapy then surgery. The interval between the beginning of pre-operative chemotherapy and surgery in group A and B was 3 to 6 months (median 3.5 months). In both groups, patients returned to chemotherapy within 3 months post-operatively.
Table 1.
Clinical characteristics
| Variables | Group A, N (%) | Group B, N (%) | P-value |
|---|---|---|---|
| n | 384 | 117 | – |
| Gender | |||
| Male | 251 (65.3%) | 75 (64.1%) | 0.809 |
| Female | 133 (34.6%) | 42 (35.9%) | |
| Age (years) | |||
| Median (range) | 68.3 (56.9) | 62.5 (47.2) | <0.01 |
| Primary tumour site | |||
| Colon | 259 (67.4%) | 95 (81.1%) | <0.01 |
| Rectum | 118 (30.7%) | 22 (18.8%) | |
| T stage | |||
| I | 8 (2.0%) | 2 (1.7%) | 0.547 |
| II | 29 (7.5%) | 14 (12.9%) | |
| III | 233 (60.6%) | 78 (66.6%) | |
| IV | 69 (17.9%) | 21 (17.9%) | |
| N stage | |||
| 0 | 115 (29.9%) | 32 (27.3%) | 0.363 |
| I | 141 (36.7%) | 38 (32.4%) | |
| II | 91 (23.6%) | 35 (30.0%) | |
| Pre-operative chemotherapy for primary cancer | 71 (18.5%) | 19 (16.2%) | 0.641 |
| Pre-operative radiotherapy for primary cancer | 56 (14.5%) | 7 (5.9%) | 0.023 |
| Synchronicity of liver metastases | |||
| Synchronous | 291 (75.7%) | 89 (76.0%) | 0.935 |
| Metachronous | 90 (23.4%) | 27 (23.1%) | |
| Number of liver metastases | |||
| 1 | 133 (34.6%) | 35 (29.9%) | 0.072 |
| 2–3 | 133 (34.6%) | 34 (29.5%) | |
| 4–5 | 67 (17.5%) | 25 (21.3%) | |
| >5 | 41 (10.1%) | 19 (16.2%) | |
| Maximum size of metastases (mm) | |||
| Median (range) | 30 (499) | 30 (149) | 0.897 |
| Location of liver metastases | |||
| Unilobar | 215 (55.9%) | 61 (52.1%) | 0.524 |
| Bilobar | 166 (43.2%) | 55 (47%) | |
| CEA at diagnosis (ng/ml) | |||
| Median (range) | 17.0 (9980) | 9.7 (5244) | 0.043 |
| Initial unresectable liver disease | 84 (21.8%) | 33 (28.2%) | 0.127 |
| Major hepatectomy | 229 (59.6%) | 71 (60.7%) | 0.395 |
| Two stage resection | 23 (6.0%) | 12 (10.3%) | 0.113 |
| Local treatment | 73 (19.0%) | 17 (14.5%) | 0.269 |
| Preoperative chemotherapy | |||
| Number of cycles | |||
| Median (range) | 6 (17) | 6 (12) | 0.441 |
| Pre-operative clinical response | 0.348 | ||
| Complete/Partial | 233 (60.7%) | 78 (66.7%) | |
| No change/Progression | 68 (17.7%) | 29 (24.7%) | |
| Post-operative chemotherapy | |||
| Number of cycles | |||
| Median (range) | 6 (24) | 6 (11) | 0.108 |
| Post-operative clinical response | 0.423 | ||
| No recurrence | 133 (34.6%) | 32 (27.4%) | |
| Recurrence/Progression | 121 (31.5%) | 27 (23.1%) |
SD, standard deviation, CEA, carcinoembryonic antigen.
Post-operative complications
Analysis of overall post-operative complications is included in Table 2. The non-tumoural liver was not significantly different between the two groups. The incidence of steatosis (33.2 versus 39.4%, P = 0.225), fibrosis (14.1 versus 9.8%, P = 0.253) and sinusoidal congestion (19.7 versus 11.3%, P = 0.072) were similar between group A and B, respectively.
Table 2.
Post-hepatectomy complications
| Variables | Group A, N (%) | Group B, N (%) | P-value |
|---|---|---|---|
| Overall complications | 114 (29.6%) | 30 (25.6%) | 0.673 |
| Overall infectious complications | 18 (4.7%) | 15 (12.8%) | <0.01 |
| Intra-abdominal abscess | 14 (3.6%) | 8 (6.8%) | 0.032 |
| Wound infection | 4 (1.1%) | 7 (5.9%) | <0.01 |
| Hepatic insufficiency | 14 (3.7%) | 1 (0.8%) | 0.101 |
| Bile leak/biloma | 28 (7.3%) | 3 (2.6%) | 0.524 |
| Pleural effusion | 19 (4.9%) | 2 (1.7%) | 0.106 |
| Ileus | 5 (1.3%) | 3 (2.6%) | 0.387 |
| Pneumonia | 8 (2.1%) | 2 (1.7%) | 0.739 |
| Haemorrhage | 6 (1.6%) | 2 (1.7%) | 0.454 |
| Sepsis | 3 (0.8%) | 0 (0%) | 0.325 |
| Arrhythmia | 5 (1.3%) | 0 (0%) | 0.204 |
| UTI | 4 (1.1%) | 0 (0%) | 0.254 |
| DVT/PE | 3 (0.8%) | 0 (0%) | 0.252 |
| SBO | 1 (0.3%) | 2 (1.7%) | 0.081 |
| Percutaneous drainage | 31 (8.1%) | 10 (8.5%) | 0.917 |
| Reoperation | 4 (1.1%) | 3 (2.6%) | 0.069 |
| Duration of hospitalization [median (range), days] | 10 (50) | 9 (35) | 0.101 |
UTI, urinary tract infection; DVT/PE, deep venous thrombosis/pulmonary embolism;
SBO, small bowel obstruction.
Outcome
Restricting survival analyses to the patients with at least 12 months of follow-up, 280 patients remained in group A and 59 patients in group B. As seen in Figures 1 and 2, the addition of peri-operative bevacizumab did not significantly impact the OS at 3 years (76.4 versus 79.8%, P = 0.334) and did not influence DFS at 3 years (7.4 versus 7.9%, P = 0.082). Univariate and multivariate analysis of prognostic factors are included respectively in Tables 3 and 4.
Figure 1.
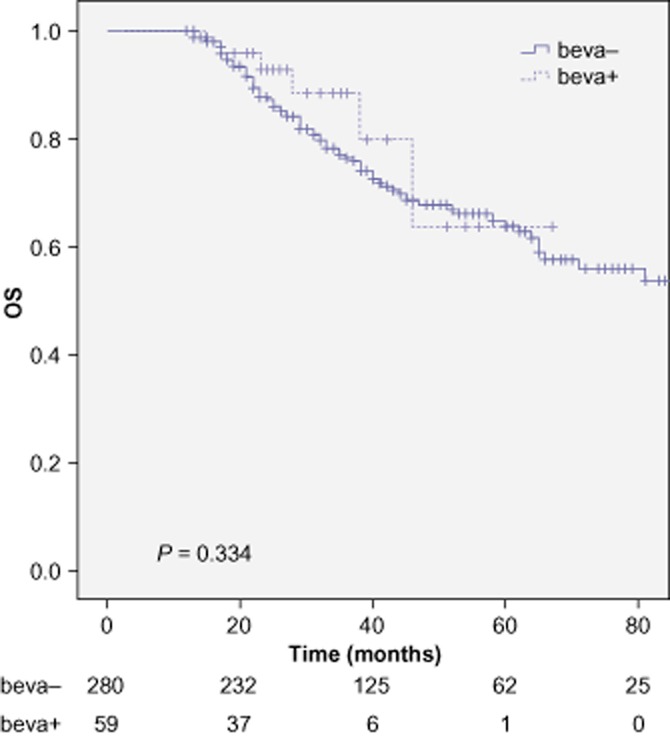
Overall survival (OS) for patients treated with peri-operative FOLFOX alone (beva-) and for patients treated with peri-operative FOLFOX + bevacizumab (beva+)
Figure 2.
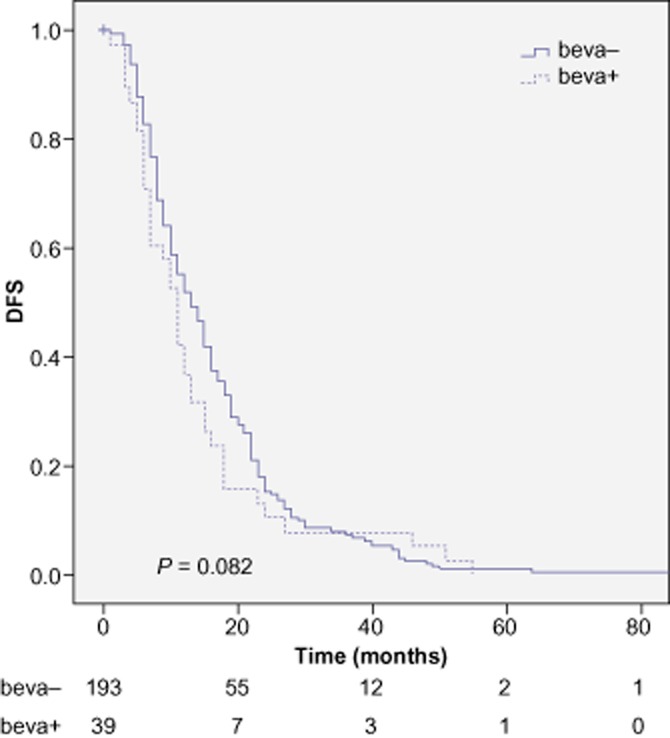
Disease-free survival (DFS) for patients treated with peri-operative FOLFOX alone (beva-) and for patients treated with peri-operative FOLFOX + bevacizumab (beva+)
Table 3.
Univariate analysis of factors associated with overall survival (OS) and disease-free survival (DFS)
| Variables | OS |
DFS |
||
|---|---|---|---|---|
| 3 years | P value | 3 year | P value | |
| Age (years) | ||||
| <70 | 73.8% | 0.812 | 63.2% | <0.01 |
| ≥70 | 67.3% | 45.5% | ||
| Gender | ||||
| Male | 65.7% | 0.371 | 53.1% | 0.898 |
| Female | 74.4% | 54.6% | ||
| Location of primary tumour | ||||
| Colon | 67.5% | 0.205 | 55.2% | 0.412 |
| Rectum | 72.3% | 49.7% | ||
| Tumour stage | ||||
| T1/T2 | 83.1% | 0.462 | 57.2% | 0.795 |
| T3/T4 | 70.2% | 54.1% | ||
| Lymph node-positive primary tumour | ||||
| No | 67.2% | 0.673 | 63.6% | 0.035 |
| Yes | 72.6% | 52.3% | ||
| Synchronicity | ||||
| No | 69.1% | 0.735 | 71.3% | 0.018 |
| Yes | 67.9% | 50.6% | ||
| CEA at diagnosis | ||||
| ≤5 | 76.5% | 0.424 | 55.2% | 0.633 |
| >5 | 65.4% | 55.7% | ||
| Number of metastases | ||||
| ≤1 | 78.2% | 0.254 | 59.1% | 0.513 |
| >1 | 64.1% | 51.6% | ||
| Number of metastases | ||||
| ≤3 | 75.5% | 0.154 | 58.3% | 0.150 |
| >3 | 61.4% | 48.9% | ||
| Maximum size of metastases (mm) | ||||
| ≤10 | 93.2% | 0.022 | 63.8% | 0.624 |
| >10 | 66.3% | 51.5% | ||
| Maximum size of metastases (mm) | ||||
| ≤30 | 68.2% | 0.926 | 55.3% | 0.457 |
| >30 | 69.3% | 55.2% | ||
| Major hepatectomy | ||||
| No | 73.8% | 0.100 | 55.4% | 0.554 |
| Yes | 67.9% | 53.2% | ||
| Curative liver resection | ||||
| No | 52.3% | 0.266 | 42.2% | 0.203 |
| Yes | 70.0% | 54.3% | ||
| Number of preoperative chemotherapy cycles | ||||
| ≤6 | 69.8% | 0.185 | 45.6% | 0.038 |
| >6 | 64.7% | 54.9% | ||
| Number of post-operative chemotherapy cycles | ||||
| ≤6 | 70.8% | 0.774 | 51.3% | 0.104 |
| >6 | 71.7% | 61.8% | ||
| Pre-operative clinical response | ||||
| Complete/Partial | 69.3% | 0.423 | 55.0% | 0.151 |
| No change/Progression | 68.1% | 39.8% | ||
| Postoperative clinical response | ||||
| No recurrence | 81.1% | <0.01 | 62.2% | 0.152 |
| Recurrence/Progression | 58.2% | 42.3% | ||
| Local treatment | ||||
| No | 65.5% | 0.027 | 57.3% | 0.175 |
| Yes | 81.4% | 42.3% | ||
| Post-operative complications | ||||
| No | 69.8% | 0.676 | 54.4% | 0.909 |
| Yes | 66.4% | 41.5% | ||
| Bevacizumab | ||||
| No | 68.4% | 0.334 | 55.8% | 0.082 |
| Yes | 68.8% | 43.4% | ||
CEA, carcinoembryonic antigen.
Table 4.
Prognostic factors associated with overall survival (OS) and disease-free survival (DFS) in multivariate analysis
| Risk factors | P-value | HR | 95% CI |
|---|---|---|---|
| Overall survival | |||
| Recurrence after completing post-operative chemotherapy | 0.052 | 1.77 | [0.97–3.23] |
| Size of metastases | 0.300 | 1.07 | [0.50–2.28] |
| Local treatment | 0.075 | 2.41 | [0.91–6.35] |
| Major hepatectomy | 0.750 | 1.12 | [0.53–2.28] |
| Bevacizumab use | 0.475 | 1.45 | [0.24–1.92] |
| Disease-free survival | |||
| Lymph node-positive primary tumour | 0.011 | 1.68 | [1.12–2.50] |
| Synchronous disease | 0.041 | 1.65 | [1.02–2.67] |
| Age | 0.113 | 1.30 | [0.93–1.82] |
| Number of pre-operative cycles | 0.798 | 1.04 | [0.72–1.50] |
| Bevacizumab use | 0.408 | 1.19 | [0.78–1.81] |
HR, hazard ratio; CI, confidence interval.
Discussion
CRC is a common cancer that often carries a poor prognosis, especially when associated with liver metastases. A complete liver resection of all metastatic disease remains the only treatment with a potential for a cure, but peri-operative chemotherapy has been found to confer a benefit on survival.15,22 The addition of a biological agent such as bevacizumab to modern cytotoxic regimens seems to improve the tumour response rate and survival in first-line therapy for metastatic CRC,13,23,24 but little evidence is available concerning the peri-operative usage of bevacizumab in the context of liver metastasectomy. However, as the benefit of bevacizumab has not been shown in the adjuvant setting in stage II or III CRC, it is not actually recommended in the setting of resected CRLM by expert panels, unless a beneficial effect was seen in the pre-operative context. The present study thus examined the effect of bevacizumab added to the peri-operative FOLFOX for resected CRLM on survival. With data collected from the international prospective database LiverMetSurvey, this work is one of the largest multi-institutional retrospective studies exploring the role of peri-operative bevacizumab in the setting of resected CRLM.
In this sudy, patients who received peri-operative FOLFOX and those who received peri-operative FOLFOX and bevacizumab were generally comparable in terms of baseline demographic and disease characteristics. The initial proportion of unresectable liver disease was comparable between the groups. The rate of major hepatectomies was also strictly similar between the two groups. As the duration of follow-up time was considerably shorter for group B, the study cohort was restricted to the patients who were followed for a minimum of 12 months. This is done in order to increase detectable endpoints in survival analyses, and to reduce bias potentially caused by short follow-up. No significant differences in OS or DFS were detected between patients receiving peri-operative chemotherapy and patients who received additional peri-operative bevacizumab. The OS rates were comparable to those reported in previous retrospective studies,25,26 whereas rates of DFS at 3 years were comparatively lower than those described in the literature.27 This may be as a result of the higher proportion of patients in the present study who presented with synchronous CRLM. Indeed, in the general CRC patient population, 20% to 34% of liver metastases are synchronous.28,29 In the present study, a high percentage of patients in both groups presented with synchronous CRLM. As synchronicity of CRLM is suggested to be associated with more aggressive disease and a worse outcome,29 this higher percentage of synchronous liver metastatic disease may explain a lower DFS.
Pre-operative chemotherapy has been linked to more frequent post-operative complications.15,30 Bevacizumab has likewise been associated with potential morbidities such as arterial and venous thromboembolism, gastrointestinal perforation, bleeding and impaired wound healing, when added to pre-operative chemotherapy.31–33 However, no significant differences were described in the literature concerning the risk of increased bleeding, wound or hepatic complications when bevacizumab was stopped at least 6 weeks before surgery.32 The present study showed that infectious complications such as wound infections were significantly more frequent in the group having received bevacizumab, but not thromboembolic and haemorrhagic complications, suggesting that the risk of infectious complications may exist with the use of biological agents. There was a trend towards less sinusoidal congestion in patients treated with bevacizumab, which may support various reports in the literature describing the protective effect of bevacizumab against sinusoidal obstruction syndrome associated with oxaliplatin-based chemotherapy.34,35
Recurrence after post-operative chemotherapy demonstrated a trend towards significance as an adverse prognostic factor for OS at multivariate analysis. Independent negative prognostic factors for DFS included primary tumour lymph node positivity and synchronous presentation of metastases. The presence of such clinicopathological factors probably insinuate more aggressive tumour biology and disseminated disease in the current cohort, and is suggestive of a less favourable prognosis. In general, these findings are in agreement with previous reports.28,36,37 After controlling for all other significant factors, the addition of bevacizumab was not significantly associated with OS and DFS in multivariate analysis.
These findings must be interpreted in light of the retrospective nature of the present study. The disparity between the number of patients and duration of follow-up time in each group may have influenced the survival analyses. In addition, the presence of a higher proportion of patients with more aggressive tumour biology may have contributed to the lack of perceptible impact from the addition of bevacizumab on survival.
In conclusion, this work has demonstrated that while bevacizumab may be important to increase the tumour response rate in metastatic CRC, its peri-operative addition to modern chemotherapy does not appear to be associated with improved global survival or survival without disease in patients with resected CRLM.
Conflicts of interest
R.A. and R.L. have received speaker's honoraria from Sanofi-Aventis, Roche and Merck-Serono. LiverMetSurvey is funded by an operating grant from Sanofi-Aventis.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Van Cutsem E, Nordlinger B, Adam R, Kohne CH, Pozzo C, Poston G, et al. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42:2212–2221. doi: 10.1016/j.ejca.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Lee WS, Yun SH, Chun HK, Lee WY, Yun HR, Kim J, et al. Pulmonary resection for metastases from colorectal cancer: prognostic factors and survival. Int J Colorectal Dis. 2007;22:699–704. doi: 10.1007/s00384-006-0218-2. [DOI] [PubMed] [Google Scholar]
- 4.Adam R, Wicherts DA, de Haas RJ, Ciacio O, Levi F, Paule B, et al. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol. 2009;27:1829–1835. doi: 10.1200/JCO.2008.19.9273. [DOI] [PubMed] [Google Scholar]
- 5.Wei AC, Greig PD, Grant D, Taylor B, Langer B, Gallinger S. Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol. 2006;13:668–676. doi: 10.1245/ASO.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 6.Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 7.Curley SA. Outcomes after surgical treatment of colorectal cancer liver metastases. Semin Oncol. 2005;32(6 Suppl. 9):S109–S111. doi: 10.1053/j.seminoncol.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 8.de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440–448. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- 9.Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. discussion 25–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez FG, Drebin JA, Linehan DC, Dehdashti F, Siegel BA, Strasberg SM. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET) Ann Surg. 2004;240:438–447. doi: 10.1097/01.sla.0000138076.72547.b1. discussion 47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–722. doi: 10.1097/01.sla.0000160703.75808.7d. discussion 22–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitry E, Fields AL, Bleiberg H, Labianca R, Portier G, Tu D, et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol. 2008;26:4906–4911. doi: 10.1200/JCO.2008.17.3781. [DOI] [PubMed] [Google Scholar]
- 13.Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 14.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 15.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nordlinger B, Van Cutsem E, Gruenberger T, Glimelius B, Poston G, Rougier P, et al. Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: recommendations from an expert panel. Ann Oncol. 2009;20:985–992. doi: 10.1093/annonc/mdn735. [DOI] [PubMed] [Google Scholar]
- 17.Lubezky N, Geva R, Shmueli E, Nakache R, Klausner JM, Figer A, et al. Is there a survival benefit to neoadjuvant versus adjuvant chemotherapy, combined with surgery for resectable colorectal liver metastases? World J Surg. 2009;33:1028–1034. doi: 10.1007/s00268-009-9945-1. [DOI] [PubMed] [Google Scholar]
- 18.Macedo LT, Lima AB, Sasse AD. Addition of bevacizumab to first-line chemotherapy in advanced colorectal cancer: a systematic review and meta-analysis, with emphasis on chemotherapy subgroups. BMC Cancer. 2012;12:89. doi: 10.1186/1471-2407-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allegra CJ, Yothers G, O'Connell MJ, Sharif S, Petrelli NJ, Colangelo LH, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol. 2011;29:11–16. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Gramont A, Van Cutsem E, Tabernero J, et al. AVANT: results from a randomized, three-arm multinational phase III study to investigate bevacizumab with either XELOX or FOLFOX4 versus FOLFOX4 alone as adjuvant treatment for colon cancer [abstract] J Clin Oncol. 2011;29(Suppl. 4):362. [Google Scholar]
- 21.2011. LiverMetSurvey International Registry [database on the Internet] Available from: http://www.livermetsurvey.org(last accessed 6 October 2013)
- 22.Ciliberto D, Prati U, Roveda L, Barbieri V, Staropoli N, Abbruzzese A, et al. Role of systemic chemotherapy in the management of resected or resectable colorectal liver metastases: a systematic review and meta-analysis of randomized controlled trials. Oncol Rep. 2012;27:1849–1856. doi: 10.3892/or.2012.1740. [DOI] [PubMed] [Google Scholar]
- 23.Cao Y, Tan A, Gao F, Liu L, Liao C, Mo Z. A meta-analysis of randomized controlled trials comparing chemotherapy plus bevacizumab with chemotherapy alone in metastatic colorectal cancer. Int J Colorectal Dis. 2009;24:677–685. doi: 10.1007/s00384-009-0655-9. [DOI] [PubMed] [Google Scholar]
- 24.Welch S, Spithoff K, Rumble RB, Maroun J. Bevacizumab combined with chemotherapy for patients with advanced colorectal cancer: a systematic review. Ann Oncol. 2010;21:1152–1162. doi: 10.1093/annonc/mdp533. [DOI] [PubMed] [Google Scholar]
- 25.Turan N, Benekli M, Koca D, Ustaalioglu BO, Dane F, Ozdemir N, et al. Adjuvant systemic chemotherapy with or without bevacizumab in patients with resected liver metastases from colorectal cancer. Oncology. 2013;84:14–21. doi: 10.1159/000342429. [DOI] [PubMed] [Google Scholar]
- 26.Liu JH, Hsieh YY, Chen WS, Hsu YN, Chau GY, Teng HW, et al. Adjuvant oxaliplatin-or irinotecan-containing chemotherapy improves overall survival following resection of metachronous colorectal liver metastases. Int J Colorectal Dis. 2010;25:1243–1249. doi: 10.1007/s00384-010-0996-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayashi M, Inoue Y, Komeda K, Shimizu T, Asakuma M, Hirokawa F, et al. Clinicopathological analysis of recurrence patterns and prognostic factors for survival after hepatectomy for colorectal liver metastasis. BMC Surg. 2010;10:27. doi: 10.1186/1471-2482-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai MS, Su YH, Ho MC, Liang JT, Chen TP, Lai HS, et al. Clinicopathological features and prognosis in resectable synchronous and metachronous colorectal liver metastasis. Ann Surg Oncol. 2007;14:786–794. doi: 10.1245/s10434-006-9215-5. [DOI] [PubMed] [Google Scholar]
- 30.Lehmann K, Rickenbacher A, Weber A, Pestalozzi BC, Clavien PA. Chemotherapy before liver resection of colorectal metastases: friend or foe? Ann Surg. 2012;255:237–247. doi: 10.1097/SLA.0b013e3182356236. [DOI] [PubMed] [Google Scholar]
- 31.Hochster HS, Hart LL, Ramanathan RK, Childs BH, Hainsworth JD, Cohn AL, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol. 2008;26:3523–3529. doi: 10.1200/JCO.2007.15.4138. [DOI] [PubMed] [Google Scholar]
- 32.Scappaticci FA, Fehrenbacher L, Cartwright T, Hainsworth JD, Heim W, Berlin J, et al. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J Surg Oncol. 2005;91:173–180. doi: 10.1002/jso.20301. [DOI] [PubMed] [Google Scholar]
- 33.Ranpura V, Hapani S, Wu S. Treatment-related mortality with bevacizumab in cancer patients: a meta-analysis. JAMA. 2011;305:487–494. doi: 10.1001/jama.2011.51. [DOI] [PubMed] [Google Scholar]
- 34.Hubert C, Sempoux C, Humblet Y, van den Eynde M, Zech F, Leclercq I, et al. Sinusoidal obstruction syndrome (SOS) related to chemotherapy for colorectal liver metastases: factors predictive of severe SOS lesions and protective effect of bevacizumab. HPB. 2013 doi: 10.1111/hpb.12047. doi: 10.1111/hpb.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klinger M, Eipeldauer S, Hacker S, Herberger B, Tamandl D, Dorfmeister M, et al. Bevacizumab protects against sinusoidal obstruction syndrome and does not increase response rate in neoadjuvant XELOX/FOLFOX therapy of colorectal cancer liver metastases. Eur J Surg Oncol. 2009;35:515–520. doi: 10.1016/j.ejso.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644–657. doi: 10.1097/01.sla.0000141198.92114.f6. discussion 57–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]


