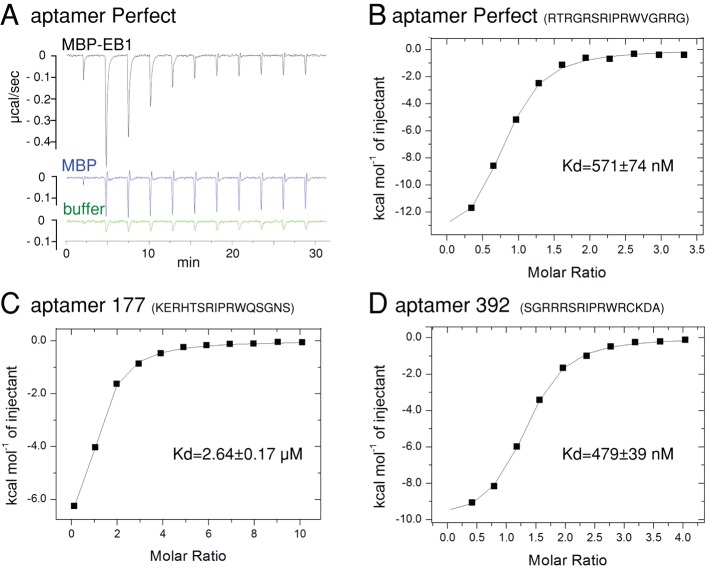
FIGURE 3:
ITC shows a high affinity of aptamers to Drosophila EB1. (A) Raw ITC data showing that aptamer Perfect interacts with MBP-fused Drosophila EB1, but not MBP, in vitro. Heat released by titrations of 100 μM aptamer Perfect into 5 μM solution of MBP-EB1, MBP, and buffer alone. Each peak corresponds to one injection. An initial smaller injection was followed by 10 injections. For MBP-EB1, the heat became smaller for each injection, as the binding site became saturated. For buffer and MBP, it stayed constant, as heat was released only from dilution of the peptide without specific binding. (B–D) Integrated heat peaks subtracted by the heat of dilution and plotted against the molar ratio of the peptide for aptamer Perfect (B), 177 (C), or 392 (D) to MBP-EB1. The line represents the fit to the single-site binding model by the Origin program.