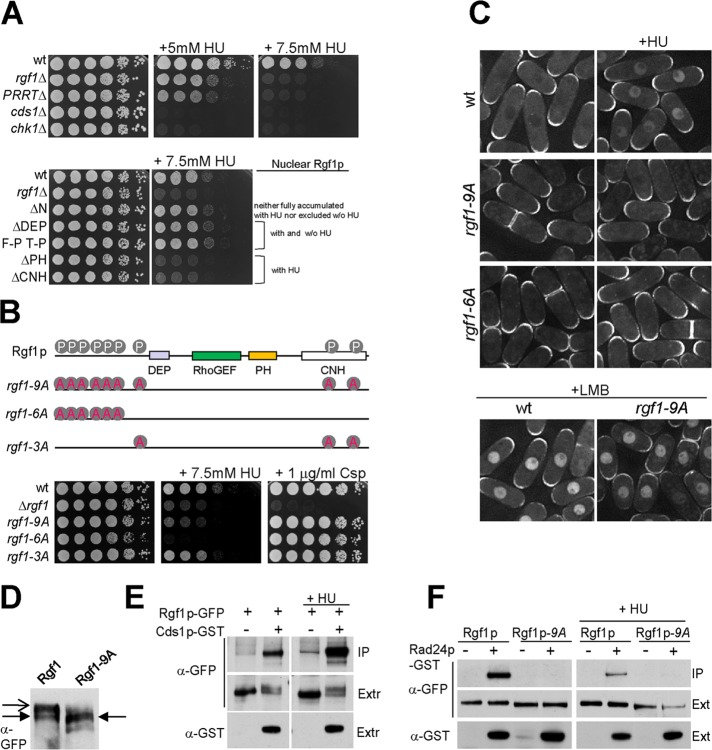
FIGURE 6:
Rgf1p is required for survival in HU. (A) For HU hypersensitivity, serial dilutions of the wild-type, rgf1Δ, rgf1ΔPTTR, cds1Δ, and chk1Δ cells were incubated at 28ºC on YES plates with no HU, 5 mM HU, or 7.5 mM HU (top). Bottom, the same type of analysis with the wild-type, rgf1Δ, Rgf1pΔN-GFP, Rgf1pΔDEP-GFP, Rgf1pFPTP-GFP, Rgf1pΔPH-GFP and Rgf1pΔCNH-GFP cells. (B) Nine putative Cds1p phosphorylation sites (RXXS) on Rgf1p are shown. Alanine substitution mutations of the nine Cds1p (S35A, S68A, S87A, S170A, S275A, S342A, S422A, S1085A, and S1322A) Rgf1p-9A sites, six Cds1p (S35A to S342A) Rgf1p-6A sites, and three Cds1p (S422A, S1085A, and S1322A) Rgf1p-3A sites were integrated chromosomally in the rgf1Δ deletion strain and expressed under the native promoter. Hypersensitivity to HU and Csp in the wild-type, rgf1Δ, Rgf1p-9A, Rgf1p-6A, and Rgf1p-3A is shown. (C) Localization of wild-type Rgf1p-GFP and the mutants rgf1Δ, Rgf1p-9A-GFP, and Rgf1-6A-GFP in untreated and 12.5 mM HU–treated cultures. Bottom, wild-type cells and Rgf1p-9A-GFP were treated with 100 ng/ml LMB for 30 min. (D) Proteins in cell extracts from Rgf1p-GFP– or Rgf1p-9A-GFP–expressing cells were separated by SDS–PAGE in the presence of 40 mM phostag and the proteins were detected by immunoblotting using anti-GFP antibodies. Closed arrow indicates major species observed in Rgf1-9A-GFP extracts. Open arrow indicates slower-migrating species observed in Rgf1p-GFP extracts. (E) GST pull-down assay showing the interaction of Rgf1p and Cds1p. Cells expressing endogenous Rgf1p-GFP and GST-Cds1p from a thiamine-regulated promoter (pREP4xGST-cds1+) or Rgf1p-GFP and the control plasmid (pREP4xGST) were grown in the absence of thiamine and incubated in the absence or presence of 12.5 mM HU for 2.5 h. The complexes precipitated with glutathione–sepharose beads were Western blotted and probed with anti-GFP antibodies to analyze Rgf1p-GFP in the immunoprecipitate (IP) and whole extract (Ext). (F) Interaction between Rgf1p and Rad24p was abolished by Rgf1p-9A. Rgf1p-GFP, Rgf1p-GFP Rad24p-GST, Rgf1p-9A-GFP, and Rgf1p-9A-GFP Rad24p-GST cells grown to mid log phase were incubated in the absence (left) or presence (right) of 12.5 mM HU for 2.5 h and lysed under native conditions. The complexes precipitated with glutathione–sepharose beads were Western blotted and probed with anti-GFP antibodies to analyze Rgf1p-GFP (IP). Whole-cell extract (Ext) fractions were assayed with anti-GFP and anti-GST antibodies.