Abstract
Objective
Estrogen-based hormone therapy (HT) attenuates abdominal fat gain after menopause, but whether HT improves abdominal fat loss during weight loss is unknown. We hypothesized that HT or a selective estrogen receptor modulator (raloxifene) would augment reductions in abdominal visceral fat during weight loss when compared to placebo, potentially increasing improvements in glucose tolerance and lipid profile.
Design and Methods
Healthy postmenopausal women (n=119; age 50–70y) underwent a 6-month weight loss (primarily exercise) intervention with randomization to raloxifene (60mg/d), HT (conjugated estrogens, 0.625mg/d), or placebo. We measured changes in total and abdominal (visceral and subcutaneous) fat mass, lipid profile, and fasting and post-challenge glucose and insulin.
Results
Neither HT nor raloxifene augmented loss of total or abdominal fat mass during exercise-induced weight loss when compared with placebo. Weight loss-induced improvements in risk factors were similar among the three groups, except for a greater reduction in fasted glucose in the HT group (difference in change [95%CI] from placebo; −0.40 [−0.76, −0.05]) and greater reductions in LDL (−0.36 [−0.63, −0.09]) and increases in HDL (0.15 [0.07, 0.24]) in both treatment groups.
Conclusions
Postmenopausal HT and raloxifene did not increase abdominal fat loss during weight loss, but did improve some cardiometabolic outcomes.
Keywords: weight loss, postmenopausal hormone therapy, cardiometabolic risk
INTRODUCTION
Excess fat stored in the abdomen, particularly in the visceral region, is associated with increased risk for heart disease, glucose intolerance, insulin resistance, Type 2 diabetes, and hypertension 1. Menopause appears to diminish the protection women are afforded from abdominal obesity prior to menopause. Evidence suggests estrogen deficiency in women after menopause promotes positive energy balance and increased fat mass, particularly in the abdominal region 2–4. Treating postmenopausal women with estrogen-based hormone therapy (HT) attenuates fat gain, when compared with placebo 5,6. Further, a large meta-analysis of randomized placebo-controlled trials demonstrated that postmenopausal HT reduced abdominal fat by 6.8% and improved cardiometabolic outcomes associated with central adiposity (e.g., insulin sensitivity, new-onset diabetes, lipids, blood pressure) 7. However, it is not known whether postmenopausal HT augments total and abdominal fat loss under conditions of negative energy balance. Moreover, weight loss itself is known to improve cardiometabolic risk factors (e.g., glucose tolerance, insulin sensitivity, lipids), but whether HT augments or attenuates weight-loss mediated improvements is not known.
Although the use estrogen-based HT after menopause for the prevention of cardiovascular disease has been the subject of much debate over the past decade, the quest to elucidate the physiological effects of estrogens remains highly important. Selective estrogen receptor modulators (SERM) were developed as an alternative postmenopausal HT because they act as both estrogen receptor agonists and antagonists, depending on the tissue. For example, raloxifene is an agonist in bone and an antagonist in breast tissue and the only SERM currently approved for the prevention of osteoporotic fractures and estrogen receptor-positive breast cancer 8. It is not known whether raloxifene acts as an estrogen receptor agonist or antagonist in adipose tissue, but raloxifene has been shown to improve some markers of cardiometabolic risk 9. Whether raloxifene augments or attenuates weight-loss mediated improvements is not known.
The primary aim of the study was to determine whether HT (oral conjugated estrogens) or a SERM (raloxifene) exaggerated abdominal fat loss during weight loss in postmenopausal women. The secondary aim was to determine whether weight-loss induced improvements in cardiometabolic outcomes were augmented or attenuated by HT or SERM treatment. To meet these aims, we induced weight loss through a 6-month program of supervised exercise training plus mild intermittent caloric restriction in postmenopausal women randomized to treatment with HT, raloxifene, or placebo. We hypothesized that reductions in total fat mass and total abdominal and visceral fat would be greater during weight loss in women treated with HT or raloxifene compared to placebo. We further hypothesized that the greater improvements in abdominal fat would augment improvements in cardiometabolic outcomes (glucose tolerance, insulin sensitivity, and lipid profiles).
METHODS AND PROCEDURES
Study participants
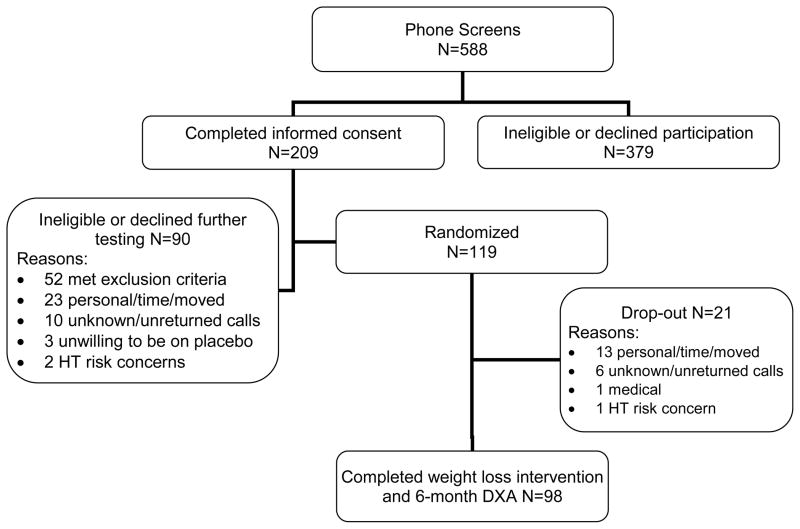
Study participants were healthy, sedentary postmenopausal women aged 50 to 70 y who had not used menopausal HT in the previous 6 months. Inclusion and exclusion criteria for the study population were previously reported 10. Briefly, women were overweight to moderately obese, non-smokers, and did not have diabetes or cardiovascular disease. Postmenopausal status was defined as the absence of menses for ≥1 year or a serum FSH >30 IU/L in women who had undergone a hysterectomy without oophorectomy. The study was approved by the Colorado Multiple Institutional Review Board and all volunteers provided written informed consent to participate. Of the 588 postmenopausal women who inquired about the study, 209 underwent informed consent and, of those, 119 were randomized into a 6-month weight loss intervention with three drug treatment arms (Figure 1).
Figure 1.
Enrollment schematic
Intervention
Study Drug
Women were randomized in a double-blinded manner to placebo, raloxifene, or HT. The HT was 0.625 mg/d conjugated estrogens (Premarin®, Pfizer Inc.); women with an intact uterus also received 5 mg/d medroxyprogesterone acetate (MPA) for 13 consecutive days trimonthly. This HT regimen was selected to minimize exposure to progestins while still protecting the endometrium. Raloxifene (Evista®, Eli Lilly) treatment was 60 mg daily and placebo treatment was a daily placebo tablet; women in these groups with an intact uterus also received cyclic placebo MPA. All study drugs and placebo tablets were purchased through a local pharmacy (Belmar Pharmacy, Lakewood, CO). Compliance to study drug was estimated from pill counts on returned bottles, study exit surveys, and serum estradiol and raloxifene concentrations measured at the baseline and 6-month study visits.
Weight Loss
All 119 women were enrolled in a 6-month weight loss intervention. Weight loss was primarily exercise-induced, but also included 1 week of caloric restriction during months 1, 3 and 5 of the supervised exercise intervention to boost weight loss. The supervised endurance exercise program was designed to increase energy expenditure by approximately 400 kcal per session. Women were asked to attend supervised exercise sessions 4 days per week, which would generate a weight loss of ~4.5 kg. They were also encouraged to do additional exercise at home. All exercise, including any done at home, was recorded in exercise logs. Mode of exercise (e.g., treadmill, elliptical, rowing ergometer), duration of activity (minutes), workload (speed, grade), and intensity (heart rate monitors, Polar Electro Inc) were used to quantify caloric expenditure for each session. Exercise intensity was progressively increased during the first few weeks until women were exercising at 70 to 80% of maximal heart rate for 45 to 60 minutes per day. Because weight loss with exercise occurs slowly 11, the 6-month program included 1-week periods of reduced-calorie diets provided by the Clinical and Translational Research Center (CTRC) Metabolic Kitchen during months 1, 3 and 5. Energy intake was reduced to 25 kcal per kg fat-free mass per day, but not less than 1200 kcal per day, with 60% of the energy as carbohydrate, 25% as protein, and 15% as fat. Of the 119 women enrolled, 98 completed the 6-month weight loss intervention and body composition assessment.
Procedures
Body composition
Total body and regional (trunk, arm, and leg) fat mass (FM) and fat-free mass (FFM) were measured by dual-energy x-ray absorptiometry (DXA) at baseline and 6 months using either a Lunar DPX-IQ (n = 72; Lunar Co., Madison, WI, software version 4.38) or a Hologic Delphi-W (n = 47; Hologic, Inc., Bedford, MA, software version 11.2) instrument. The recommendations of the manufacturers were used to define the trunk, arm and leg regions. The use of two DXA instruments could not be avoided, but each participant had baseline and 6-month measurements on the same instrument. The within-instrument coefficients of variation (CV) for FM and FFM were 1.1±0.9% and 0.5±0.4%, respectively.
Abdominal visceral and subcutaneous fat areas were determined by computed tomography (CT) using a General Electric (Waukesha, WI) High Speed CT. Single axial CT images (120 kVp, 200–300 mAs, 10-mm slice thickness) were acquired at the levels of the L2-L3 and L4-L5 intervertebral spaces; the areas for the 2 slices were averaged. Adipose tissue areas were determined using a CT intensity range from image-generated histograms of adipose and soft tissue regions. The abdominal visceral fat areas (VFA; cm2) were manually outlined by tracing the muscles of the abdominal wall. Abdominal subcutaneous fat areas (SFA; cm2) were calculated by subtracting the visceral fat areas from the total abdominal fat area.
Oral Glucose tolerance test (OGTT)
A 75-g OGTT was administered in the morning after an overnight fast. Blood samples were obtained before and 30, 60, 90, and 120 min after glucose ingestion for glucose and insulin determinations. The total areas under the glucose (GLUA) and insulin (INSA) curves were calculated using the trapezoidal rule. The INSA and fasted insulin (INS0) were used as indices of hyperinsulinemia and the product of the insulin and glucose areas (INSA x GLUA) was calculated as an estimate of peripheral insulin resistance (i.e., Matsuda Index) as previously described 12. The areas and products were left as conventional (non SI) units for consistency with previous studies.
Hormones and metabolites
Blood samples were stored at −80° C and analyzed in batch by the CTRC Core Laboratory. Serum insulin concentrations were determined with a double-antibody radioimmunoassay (Diagnostic Systems Laboratory, Webster, TX). Serum glucose was measured using a hexokinase assay on a Cobra Mira Plus instrument (Roche Diagnostic Systems, Indianapolis, IN). Intra- and inter-assay coefficients of variation were 5.2% and 9.8% for insulin measurements; and 1.1% and 3.6% for glucose determinations.
Blood lipids and lipoproteins
Measurements of serum lipid and lipoprotein concentrations were done by the CTRC Core Laboratory. Total cholesterol (C), High-density lipoprotein-C (HDL-C) and triglycerides (TG) were measured by automated enzymatic commercial kits on a Cobra Mira Plus instrument (Roche Diagnostic Systems, Indianapolis, IN). Intra- and inter-assay coefficients of variation were as follows: 1) TC, 5.1% and 2.4%; 2) TG, 1.4% and 3.3%; 3) HDL, 4.5% and 2.9%. Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald equation.
Statistics
Statistical power was based on a previous study wherein exercise-induced fat loss was preserved by HT 13. In that study, the 6-month changes in trunk fat in the HT group and control group during the period of weight regain following completion of the exercise intervention were 0.3 ± 1.6 kg and 1.5 ± 1.7 kg, respectively, suggesting that 30 women per group would provide 80% power to detect a 1.2 kg difference between groups in a two-sided level α=0.05 t test.
Participant characteristics at baseline were compared across groups by one-way ANOVA for continuous measures or chi-squared tests of equal proportions for categorical variables. The effects of treatment and weight loss were addressed using a statistical model that regressed the change from baseline in the outcome measure on the corresponding baseline value and an indicator for treatment group. Baseline was included to increase the efficiency (increase power) of the estimates. The model was parameterized so that 3 statistical tests (contrasts) could be evaluated to compare the effect of HT vs. placebo, raloxifene vs. placebo, and the average of the two treatments vs. placebo. Both absolute and relative (i.e., percent of total fat mass) changes in regional adiposity were evaluated but results did not differ, so only absolute changes are presented. Likewise, the results of intent-to-treat and compliance (drug and exercise) analyses did not differ appreciably, so only the intent-to-treat results are presented. Data are presented as mean±SD unless otherwise specified.
RESULTS
Study cohort
The three groups of postmenopausal women randomized to HT, raloxifene or placebo, were similar with respect to age, number of years since menopause, hysterectomy status, past exposure to HT, aerobic fitness, and degree of obesity (Table 1). Of the 119 women randomized into the study, 98 women completed the exercise and weight loss intervention and 6-month follow-up body composition assessment by DXA (primary outcome). The 21 non-completers did not significantly differ from the 98 completers in any of the outcomes (data not shown) with the exception that the drop-outs had a significantly (p<0.05) larger waist girth (97±13 vs 91±12cm) at baseline. Of the 98 completers, 93 had paired OGTT and lipid profiles and 85 had paired abdominal CT data. Missing follow-up data were primarily due to the inability to schedule tests within one month of completing the weight loss intervention. Additional CT data were lost to incorrect scan levels being obtained at either baseline or follow-up. Exercise volume, intensity and energy expenditure were similar among the 3 groups (Table 2). At study entry, groups were similar for total adiposity and regional adipose deposition, with a propensity for abdominal adiposity as evidenced by the high waist girths and visceral fat areas (Table 3).
Table 1.
Baseline characteristics of postmenopausal women randomized to placebo, raloxifene, or hormone therapy (HT).
| Treatment Group | |||
|---|---|---|---|
| placebo n = 38 | raloxifene n =38 | HT n =43 | |
| Age, yr | 56±5 | 56±4 | 56±4 |
| Age at menopause, yr | 47±6 | 47±6 | 47±7 |
| Time since menopause, yr | 9.2±8.3 | 8.5±7.3 | 9.0±7.9 |
| Hysterectomy, n (%) | 13 (34%) | 13 (34%) | 14 (33%) |
| Time since last HT use, yr (n) | 3.7±5.0 (28) | 2.6±3.3 (21) | 2.7±4.3 (26) |
| Duration of previous HT use, yr | 4.7±7.9 | 5.8±7.0 | 4.5±4.8 |
| VO2 peak, L/min | 1.76±0.25 | 1.78±0.36 | 1.69±0.25 |
| Height, cm | 164.0±6.2 | 163.2±8.1 | 163.2±6.9 |
| Weight, kg | 80.8±12.3 | 81.4±13.2 | 78.7±12.6 |
| BMI, kg/m2 | 30.0±4.1 | 30.6±4.7 | 29.7±4.5 |
Table 2.
Volume of exercise performed during the 6-month exercise-induced weight loss intervention in women randomized to placebo, raloxifene, or hormone therapy (HT) who completed the 6-month intervention.
| Treatment Group
|
|||
|---|---|---|---|
| Placebo n=29 | Raloxifene n=34 | HT n=35 | |
| Days per wk | 3.1±1.0 | 3.3 ± 1.1 | 3.0 ± 1.0 |
| Minutes per wk | 157.8±68.4 | 166.4±64.0 | 153.7±55.9 |
| Average heart rate (beats/min) | 133.4 ± 12.3 | 134.2±12.9 | 132.7±10.8 |
| Exercise energy expenditure (kcal/wk) | 1219.2±703.1 | 1266.9±608.4 | 1214.2±537.5 |
Table 3.
Baseline (mean±SD) and comparison of change in 6-month (difference [95%CI]) body composition in postmenopausal women treated with placebo, raloxifene, or hormone therapy (HT) who completed the intervention.
| Baseline | Difference in change from Placebo | ||||
|---|---|---|---|---|---|
|
| |||||
| Placebo | Raloxifene | HT | Raloxifene | HT | |
| Anthropometric, n | 28 | 34 | 34 | 34 | 34 |
| Waist, cm | 90.8±13.0 | 92.5±10.4 | 90.9±12.4 | −1.9 [−4.6, 0.8] | 0.3 [−2.4, 3.0] |
| Hip, cm | 111.4±10.1 | 112.9±10.7 | 110.4±9.5 | −1.5 [−4.1, 1.0] | −0.8 [−3.4, 1.7] |
| Thigh, cm | 56.5±5.7 | 56.6±5.8 | 56.1±5.2 | −0.4 [−1.9, 1.1] | −0.0 [−1.5, 1.5] |
| DXA, n | 29 | 34 | 35 | 34 | 35 |
| Weight, kg | 79.5±11.7 | 81.4±13.0 | 79.2±12.9 | −0.7 [−2.4, 1.1] | −0.0 [−1.7, 1.7] |
| FFM, kg | 44.0±5.3 | 45.4±5.9 | 44.9±5.2 | −0.5 [−1.2, 0.2] | −0.1 [−0.8, 0.6] |
| FM, kg | 35.5±8.0 | 36.0±9.2 | 34.3±8.5 | −0.1 [−1.8, 1.6] | 0.0 [−1.7, 1.7] |
| Trunk FM, kg | 17.3±4.2 | 17.8±4.1 | 16.8±4.4 | −0.0 [−1.0, 1.0] | 0.1 [−0.9, 1.1] |
| Leg FM, kg | 13.4±3.3 | 13.2±4.74 | 12.8±3.7 | −0.1 [−0.7, 0.6] | −0.2 [−0.9, 0.4] |
| CT, n | 28 | 27 | 30 | 27 | 30 |
| AFA, cm2 | 473±109 | 487±143 | 479±134 | −13.6 [−41.3, 14.1] | −12.3 [−39.2, 14.7] |
| SFA, cm2 | 355±91 | 361±117 | 344±97 | −10.9 [−30.6, 8.8] | −10.0 [−29.2, 9.2] |
| VFA, cm2 | 118±55 | 126±40 | 135±55 | −2.3 [−13.3, 8.8] | −1.6 [−12.5, 9.2] |
AFA, abdominal fat area; FM, fat mass; FFM, fat-free mass; SFA, subcutaneous fat area, VFA, visceral fat area.
Body composition changes following intervention
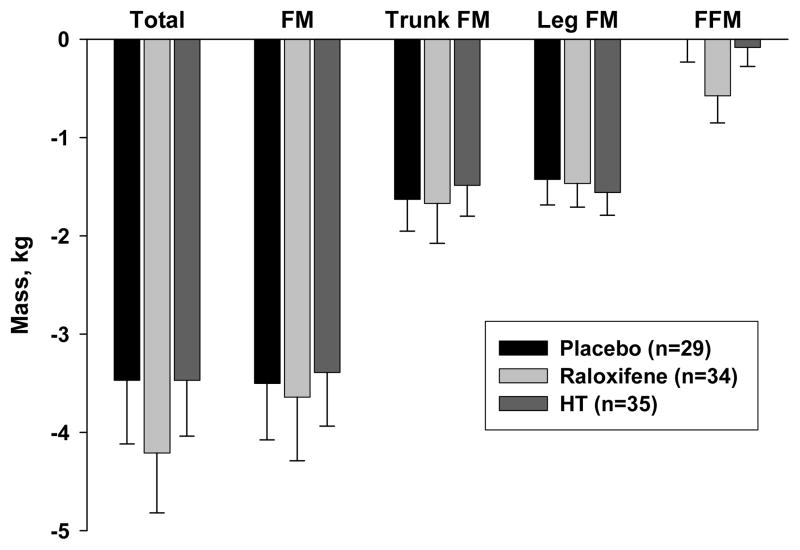
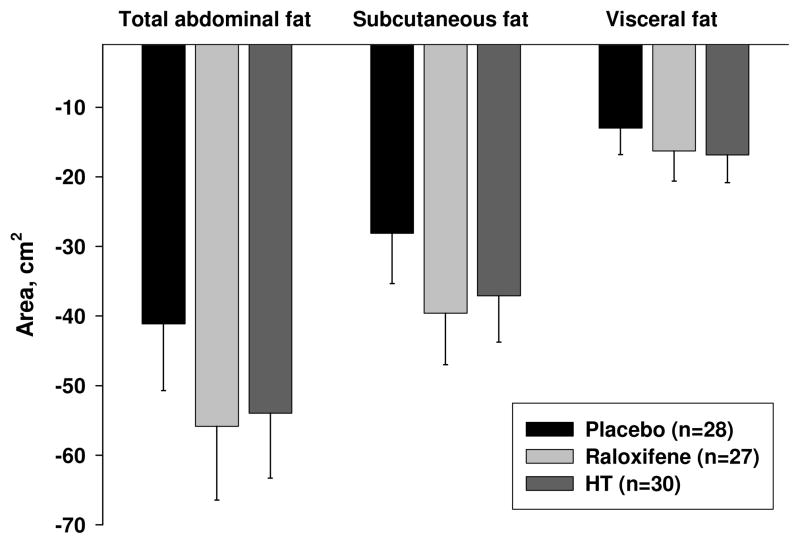
For all 3 groups weight loss and fat loss during the 6-month exercise intervention averaged −3.7±3.4 kg and −3.5±3.4 kg, respectively (Table 3, Figure 2). Trunk and leg fat decreased by −1.6±2.0 kg and −1.5±1.4 kg, respectively, while fat-free mass was maintained (−0.2±1.4 kg). Fat loss from the abdominal compartment (as measured by CT) was highly variable, with an average decrease in fat area of −34.9±37.6 cm2 from the subcutaneous region and −15.4±21.3 cm2 from the visceral region (Table 3, Figure 3). There were no differences in total body and regional fat loss among the 3 drug treatment groups.
Figure 2.
Six-month changes (n=98; mean±SE) in total and regional mass among postmenopausal women randomized to treatment with placebo, raloxifene, or estrogen-based hormone therapy (HT) during an exercise-induced weight loss intervention.
Figure 3.
Six-month changes in CT abdominal fat (n=85; mean±SE) among postmenopausal women randomized to treatment with placebo, raloxifene, or estrogen-based hormone therapy (HT) during an exercise-induced weight loss intervention.
Cardiometabolic changes following intervention
Exercise-induced weight loss resulted in significant decreases in fasted, 2-hour, and area under the curve for glucose and insulin, but the improvements were similar among the HT, raloxifene and placebo groups (Tables 4 and 5). Insulin sensitivity (INSA*GLUA) was equally improved in all groups after 6 months of weight loss. The only significant difference among the groups was a greater reduction in fasted glucose in HT compared with placebo (p<0.05).
Table 4.
Baseline (mean±SD) and comparison of change in 6-month (difference [95%CI]) oral glucose tolerance test (OGTT) and lipid data in postmenopausal women treated with placebo, raloxifene, or hormone therapy (HT) who completed the intervention and OGTT visit.
| Baseline | Difference in change from Placebo | ||||
|---|---|---|---|---|---|
|
| |||||
| Placebo | Raloxifene | HT | Raloxifene | HT | |
| OGTT, n | 27 | 33 | 33 | 33 | 33 |
| GLU0, mmol/L | 5.0±0.5 | 5.2±1.4 | 5.3±0.7 | −0.06 [−0.41, 0.30] | −0.40 [−0.76, −0.05]* |
| GLU120, mmol/L | 6.7±2.2 | 7.5±3.5 | 7.2±2.5 | 0.70 [−0.27, 1.66] | 0.56 [−0.40, 1.52] |
| GLUA, *103 | 16.5±3.3 | 17.7±5.9 | 17.0±3.9 | 0.75 [−0.81, 2.30] | 0.46 [−1.08, 2.01] |
| INS0, pmol/L | 58.5±24.4 | 60.7±32.6 | 73.3±38.0 | −10.0 [−24.0, 3.0] | −3.4 [−16.6, 9.7] |
| INS120, pmol/L | 328±253 | 404±294 | 421±306 | −36 [−233, 162] | 86 [−111, 284] |
| INSA, *103 | 6.3±3.2 | 6.8±3.7 | 7.0±4.0 | −0.23 [−1.90, 1.43] | 0.59 [−1.07, 2.26] |
| INSA* GLUA,*108 | 1.0±0.6 | 1.2±0.7 | 1.2±0.6 | 0.03 [−0.32, 0.37] | 0.18 [−0.17, 0.53] |
| Lipid profile, n | 27 | 33 | 33 | 33 | 33 |
| Total C, mmol/L | 5.34±0.89 | 5.13±0.76 | 5.08±0.71 | −0.17 [−0.51, 0.16] | −0.14 [−0.48, 0.19] |
| TG, mmol/L | 1.99±1.18 | 1.84±0.63 | 2.02±0.68 | 0.11 [−0.21, 0.43] | 0.28 [−0.05, 0.60] |
| HDL-C, mmol/L | 1.33±0.44 | 1.26±0.27 | 1.17±0.38 | 0.16 [0.06, 0.25]* | 0.15 [0.05, 0.25]* |
| LDL-C, mmol/L | 3.33±0.79 | 3.24±0.72 | 3.21±0.77 | −0.34 [−0.65, −0.03]* | −0.37 [−0.68, −0.06]* |
0 subscript, fasting (0 min); 120 subscript, post-glucose challenge (120 min); A, area under the curve; C, cholesterol; GLU, glucose; HDL, high density lipoprotein; INS, insulin; LDL, low density lipoprotein;
p<0.05.
Table 5.
Six-month change (mean±SD) oral glucose tolerance test (OGTT) and lipid data in postmenopausal women treated with placebo, raloxifene, or hormone therapy (HT) who completed the intervention and OGTT visit.
| 6-month Change | |||
|---|---|---|---|
|
| |||
| Placebo | Raloxifene | HT | |
| OGTT, n | 27 | 33 | 33 |
| GLU0, mmol/L | 0.03±0.52 | −0.02±0.85 | 0.35±0.61* |
| GLU120, mmol/L | −0.50±2.13 | −0.04±2.30 | −0.09±1.74 |
| GLUA, *103 | −0.86±2.42 | −0.26±3.65 | −0.46±2.79 |
| INS0, pmol/L | −5.57±25.2 | −16.5±26.2 | −15.2±32.9 |
| INS120, pmol/L | −51.0±181.5 | −79.8±233.9 | 43.5±567.3 |
| INSA, *103 | −0.92±2.24 | −1.17±3.13 | −0.35±3.89 |
| INSA* GLUA,*107 | −2.16±4.12 | −2.05±6.31 | −0.52±8.60 |
| Lipid profile, n | 27 | 33 | 33 |
| Total C, mmol/L | −0.14±0.85 | −0.23±0.62 | −0.18±0.68 |
| TG, mmol/L | −0.26±0.47 | −0.11±0.58 | 0.02±0.83 |
| HDL-C, mmol/L | −0.09±0.27 | 0.09±0.17* | 0.12±0.22* |
| LDL-C, mmol/L | 0.03±0.74 | −0.28±0.59* | −0.30±0.61* |
0 subscript, fasting (0 min); 120 subscript, post-glucose challenge (120 min); A, area under the curve; C, cholesterol; GLU, glucose; HDL, high density lipoprotein; INS, insulin; LDL, low density lipoprotein;
p<0.05, different from placebo.
In general, exercise-induced weight loss improved lipid profiles, but effects differed among the three treatment groups (Tables 4 and 5). Compared with placebo, LDL-cholesterol was decreased (p<0.05) and HDL-cholesterol increased (p<0.05) in the HT and raloxifene groups following intervention; the decrease in total cholesterol was similar for all groups. There was a non-significant trend (p=0.09) for HT to attenuate the weight loss-induced decrease in fasting TG observed in the placebo group.
DISCUSSION
To our knowledge, this was the first randomized, controlled trial in postmenopausal women to determine the effects of HT and raloxifene on regional fat distribution and cardiometabolic risk (glucose tolerance and lipid profiles) in response to exercise-induced weight loss. In contrast to the hypothesis, neither HT nor raloxifene exaggerated the decrease in total or abdominal fat mass during exercise-induced weight loss when compared with placebo. Treatment with either HT or raloxifene during weight loss had some additional beneficial effects on cardiometabolic outcomes when compared with placebo. HT, but not raloxifene, improved fasted glucose compared to placebo. LDL- and HDL-cholesterol were also improved to a greater extent in response to HT or raloxifene when compared with placebo.
Energy imbalance, fat accumulation, and estrogens
Several lines of evidence suggest that estrogen deficiency disrupts energy balance in a manner that increases propensity for weight gain. Preclinical studies have demonstrated an accelerated weight gain following ovariectomy, which is prevented with estradiol or raloxifene administration 14–16. Menopause also appears to trigger an energy imbalance that increases propensity for weight and fat gain 2–4. The menopause-related change in energy balance appears to be mediated, at least in part, by declines in ovarian hormones because GnRH agonist suppression of ovarian function has been shown to increase fat mass 17,18. Whether the energy imbalance observed during estrogen deficiency can be explained by decreases in energy expenditure or an increase in energy intake remains unclear. We previously found that sex hormone suppression caused a decrease in resting energy expenditure 19. It is not known whether this was specifically related to the suppression of ovarian estrogen secretion, but exogenous estrogen administration to postmenopausal women has been shown to attenuate weight and fat gain 5,6. Importantly, a meta-analysis of randomized trials suggests HT, compared to placebo or no treatment, reduces abdominal adiposity and improves markers of cardiometabolic risk 7. However, previous studies were conducted in postmenopausal women not actively losing weight so prior to our study it was not known whether exogenous estrogen would augment fat loss (particularly in the abdominal region) under conditions of negative energy imbalance.
SERMs interact with estrogen receptors but can have either agonist or antagonist effects in different tissues. Raloxifene is reported to act as an agonist in bone, serum lipids, and arterial vasculature and as an antagonist in breast and uterine tissues. Whether raloxifene is an estrogen receptor agonist or antagonist in adipose tissue is not yet clear, but studies of ovariectomized rats suggest that raloxifene, like estrogen, attenuates body fat accumulation 20. Likewise, raloxifene was shown to attenuate fat gain in postmenopausal women 21,22. Nevertheless, raloxifene did not augment fat loss in the present study.
It is not clear why HT and raloxifene would attenuate fat gain during positive energy balance but have no effect on fat loss during negative energy balance. However, it could be argued that the lack of an effect of estrogens or raloxifene on total or regional fat loss was not surprising given that weight loss was achieved primarily through an exercise-induced increase in energy expenditure. If the mechanism by which estrogens attenuate fat gain during positive energy imbalance is through an increase in energy expenditure, it is possible that our exercise-related increase in energy expenditure masked an effect of estrogens on fat loss. Similarly, regarding the lack of an effect of HT on regional changes in adiposity, it is possible that exercise enhanced the reduction in abdominal fat, possibly via sympathetic nervous system activity, and masked potential effects of HT. Verification of this hypothesis would require a comparison of the effects of HT on reductions in regional fat in response to diet- versus exercise-induced weight loss. The different effects of estrogens under conditions of negative, compared to positive, energy imbalance suggests a mechanism that protects against weight gain, but does not influence weight loss.
Exogenous estrogens and cardiometabolic risk
Estrogen-based HT has been shown to increase 23–25, not change 26,27, or decrease 28 the glucoregulatory action of insulin in postmenopausal women. Inconsistency among studies is likely due to widely varying types of HT regimens (e.g. varying duration of treatment, dose, route of administration, and opposition by progestins) and concurrent changes in body composition. Our previous studies of acute estrogen administration suggest estrogens can improve insulin-mediated glucose uptake in the absence of changes in body composition 29. However, the current study suggests that, in the context of equal weight loss among treatment groups, HT does not augment improvements in insulin action. On the other hand, there was a 7% (0.35 mmol/L, 6 mg/dL) reduction in fasting glucose in response to HT. This is consistent with previous observations that both oral conjugated estrogens 30,31 and transdermal estradiol 32 reduce fasting glucose in postmenopausal women. While fasting glucose may not be linearly related to cardiovascular outcomes, these small improvements were sufficient to restore fasting glucose to the low risk range (3.9–5.6 mmol/L) for these women 33. Previous studies of postmenopausal women randomized to raloxifene or placebo observed no change 34 or worsened 35 insulin action following raloxifene treatment. However, in the current study raloxifene did not diminish weight loss-induced improvements in insulin action.
Oral conjugated estrogens have generally been shown to decrease total and LDL-cholesterol and increase HDL-cholesterol and triglycerides in postmenopausal women 7,36–38. Raloxifene was also shown to improve total, HDL- and LDL-cholesterol 9. Exercise-induced weight loss can similarly improve lipid profiles, but with the added benefit of reducing serum triglycerides 39. Our data suggest that both HT and raloxifene further augment weight loss-induced decreases in LDL-cholesterol and increases in HDL-cholesterol, but tend to lessen improvements in triglyceride. The average treatment-related (i.e., difference from placebo) increase in HDL of 0.15 mmol/L (5.8 mg/dL) and decrease in LDL of 0.36 mmol/L (13.9 mg/dL) are clinically meaningful 33 in this population and consistent with previous studies of HT treatment without exercise or weight loss 7,9.
Limitations
It is not known whether our results were affected by the type of HT or SERM used in the study. Because we used oral conjugated estrogens in combination with MPA, it is possible that the anti-estrogenic effects of the progesterone attenuated the effects of the estrogens. However, the MPA was administered only trimonthly and only in those women with an intact uterus, so any effect of MPA was likely minimal. A different dose and/or route of administration (e.g., transdermal) may also have had more of an effect on body composition, but the oral dose we used was previously found to attenuate fat accumulation in postmenopausal women 7. It is not yet known whether raloxifene acts primarily as an estrogen agonist or antagonist in adipose tissue; it is possible that another SERM would have different effects on adipose tissue loss during negative energy balance.
It is also not known whether our results were affected by the type of weight loss intervention used in the study. We induced weight loss primarily through an increase in energy expenditure (daily exercise) with only a small decrease in energy intake (intermittent caloric restriction). It is possible that a greater emphasis on dietary restriction would have brought about greater improvements in fasting glucose, and LDL-cholesterol, compared to exercise-induced weight loss 40. Conversely, diet-induced weight loss might have had less impact on abdominal adiposity and insulin sensitivity than was observed with exercise-induced weight loss 40. Such differences might have altered the impact of HT and raloxifene on cardiometabolic improvements.
In summary, exercise plus intermittent caloric restriction effectively reduced fat mass and improved cardiometabolic outcomes in postmenopausal women irrespective of hormone treatment. Neither HT nor raloxifene augmented abdominal fat loss, but improvements in some indicators of cardiometabolic risk were exaggerated by HT and raloxifene treatment, independent of fat loss. The apparent lack of an effect of HT or raloxifene to amplify reductions in abdominal adiposity during weight loss is seemingly in contrast with the effect of these treatments to minimize gains in abdominal adiposity in postmenopausal women who were weight stable or in mild positive energy balance. Better understanding of the mechanisms underlying the effects of estrogens on adipose tissue and energy metabolism are needed to explain these discrepant findings.
What is already known about this subject
Postmenopausal women treated with estrogen-based hormone therapy (HT) gain less fat, particularly in the abdominal region, than those treated with placebo.
Postmenopausal HT improves insulin sensitivity and lipid profiles, when compared with placebo.
What this study adds
HT does not augment abdominal fat loss during weight loss.
HT augments improvements in fasting glucose, HDL and LDL during weight loss.
A selective estrogen receptor modulator (raloxifene) does not augment abdominal fat loss during weight loss but augments improvements in HDL and LDL, when compared with placebo.
Acknowledgments
The authors wish to thank the staffs of the University of Colorado Anschutz Medical Campus Clinical and Translational Research Center (CTRC), Department of Radiology, and Energy Balance Core of the Nutrition and Obesity Research Center (NORC) for their assistance in conducting this study. The authors would also like to thank the members of their research group for carrying out the day-to-day activities of the project and the study volunteers for their time and efforts. All authors made significant contributions to the study. WMK conceived study design, data analysis and interpretation. RV and WG carried out the intervention, data collection, and interpretation. PW and JK performed the statistical analysis and interpretation. CJ and RS provided data and safety monitoring support. All authors were involved in writing the paper and had final approval of the submitted and published versions. The following awards from the National Institutes of Health supported this research: R01 AG018198, K01 AG019630, R01 AG077992, P30 DK048520, UL1 TR000154
Footnotes
COMPETING INTERESTS
There are no conflicts of interest to disclose.
Clinical Trials #: NCT00149604
References
- 1.Björntorp P. Body fat distribution, insulin resistance, and metabolic diseases. Nutrition. 1997;13(9):795–803. doi: 10.1016/s0899-9007(97)00191-3. [DOI] [PubMed] [Google Scholar]
- 2.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes. 2008;32(6):949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lobo RA. Metabolic syndrome after menopause and the role of hormones. Maturitas. 2008;60(1):10–18. doi: 10.1016/j.maturitas.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Polotsky HN, Polotsky AJ. Metabolic implications of menopause. Semin Reprod Med. 2010;28(5):426–434. doi: 10.1055/s-0030-1262902. [DOI] [PubMed] [Google Scholar]
- 5.Kristensen K, Pedersen SB, Vestergaard P, Mosekilde L, Richelsen B. Hormone replacement therapy affects body composition and leptin differently in obese and non-obese postmenopausal women. J Endocrinol. 1999;163(1):55–62. doi: 10.1677/joe.0.1630055. [DOI] [PubMed] [Google Scholar]
- 6.Gambacciani M, Ciaponi M, Cappagli B, De Simone L, Orlandi R, Genazzani AR. Prospective evaluation of body weight and body fat distribution in early postmenopausal women with and without hormonal replacement therapy. Maturitas. 2001;39:125–132. doi: 10.1016/s0378-5122(01)00194-3. [DOI] [PubMed] [Google Scholar]
- 7.Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab. 2006;8(5):538–554. doi: 10.1111/j.1463-1326.2005.00545.x. [DOI] [PubMed] [Google Scholar]
- 8.Gizzo S, Saccardi C, Patrelli TS, Berretta R, Capobianco G, Di Gangi S, et al. Update on raloxifene: mechanism of action, clinical efficacy, adverse effects, and contraindications. Obstet Gynecol Surv. 2013;68(6):467–481. doi: 10.1097/OGX.0b013e31828baef9. [DOI] [PubMed] [Google Scholar]
- 9.Collins P, Mosca L, Geiger MJ, Grady D, Kornitzer M, Amewou-Atisso MG, et al. Effects of the selective estrogen receptor modulator raloxifene on coronary outcomes in the Raloxifene Use for The Heart trial: results of subgroup analyses by age and other factors. Circulation. 2009;119(7):922–930. doi: 10.1161/CIRCULATIONAHA.108.817577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gozansky WS, Van Pelt RE, Jankowski CM, Schwartz RS, Kohrt WM. Protection of bone mass by estrogens and raloxifene during exercise-induced weight Loss. J Clin Endocrinol Metab. 2005;90(1):52–59. doi: 10.1210/jc.2004-0275. [DOI] [PubMed] [Google Scholar]
- 11.Donnelly JE, Smith BK. Is exercise effective for weight loss with ad libitum diet? Energy balance, compensation, and gender differences. Exerc Sport Sci Rev. 2005;33(4):169–174. doi: 10.1097/00003677-200510000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Van Pelt RE, Jankowski CM, Gozansky WS, Schwartz RS, Kohrt WM. Lower-body adiposity and metabolic protection in postmenopausal women. J Clin Endocrinol Metab. 2005;90(8):4573–4578. doi: 10.1210/jc.2004-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohrt WM, Ehsani AA, Birge SJ., Jr HRT preserves increases in bone mineral density and reductions in body fat after a supervised exercise program. J Appl Physiol. 1998;84(5):1506–1512. doi: 10.1152/jappl.1998.84.5.1506. [DOI] [PubMed] [Google Scholar]
- 14.Witte MM, Resuehr D, Chandler AR, Mehle AK, Overton JM. Female mice and rats exhibit species-specific metabolic and behavioral responses to ovariectomy. Gen Comp Endocrinol. 2010;166(3):520–528. doi: 10.1016/j.ygcen.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogers NH, Perfield JW, 2nd, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150(5):2161–2168. doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorzek JF, Hendrickson KC, Forstner JP, Rixen JL, Moran AL, Lowe DA. Estradiol and tamoxifen reverse ovariectomy-induced physical inactivity in mice. Med Sci Sports Exerc. 2007;39(2):248–256. doi: 10.1249/01.mss.0000241649.15006.b8. [DOI] [PubMed] [Google Scholar]
- 17.Revilla R, Revilla M, Villa LF, Cortes J, Arribas I, Rico H. Changes in body composition in women treated with gonadotropin- releasing hormone agonists. Maturitas. 1998;31(1):63–68. doi: 10.1016/s0378-5122(98)00080-2. [DOI] [PubMed] [Google Scholar]
- 18.Yamasaki H, Douchi T, Yamamoto S, Oki T, Kuwahata R, Nagata Y. Body fat distribution and body composition during GnRH agonist therapy. Obstet Gynecol. 2001;97:338–342. doi: 10.1016/s0029-7844(00)01181-9. [DOI] [PubMed] [Google Scholar]
- 19.Day DS, Gozansky WS, Van Pelt RE, Schwartz RS, Kohrt WM. Sex hormone suppression reduces resting energy expenditure and {beta}-adrenergic support of resting energy expenditure. J Clin Endocrinol Metab. 2005;90(6):3312–3317. doi: 10.1210/jc.2004-1344. [DOI] [PubMed] [Google Scholar]
- 20.Sato M, Rippy MK, Bryant HU. Raloxifene, tamoxifen, nafoxidine, or estrogen effects on reproductive and nonreproductive tissues in ovariectomized rats. FASEB J. 1996;10(8):905–912. doi: 10.1096/fasebj.10.8.8666168. [DOI] [PubMed] [Google Scholar]
- 21.Tommaselli GA, Di Carlo C, Di Spiezio Sardo A, Bifulco G, Cirillo D, Guida M, et al. Serum leptin levels and body composition in postmenopausal women treated with tibolone and raloxifene. Menopause. 2006;13(4):660–668. doi: 10.1097/01.gme.0000227335.27996.d8. [DOI] [PubMed] [Google Scholar]
- 22.Francucci CM, Daniele P, Iori N, Camilletti A, Massi F, Boscaro M. Effects of raloxifene on body fat distribution and lipid profile in healthy post-menopausal women. J Endocrinol Invest. 2005;28(7):623–631. doi: 10.1007/BF03347261. [DOI] [PubMed] [Google Scholar]
- 23.Cucinelli F, Paparella P, Soranna L, Barini A, Cinque B, Mancuso S, et al. Differential effect of transdermal estrogen plus progestagen replacement therapy on insulin metabolism in postmenopausal women: relation to their insulinemic secretion. Eur J Endocrinol. 1999;140:215–223. doi: 10.1530/eje.0.1400215. [DOI] [PubMed] [Google Scholar]
- 24.O’Sullivan AJ, Ho KKY. A comparison of the effects of oral and transdermal estrogen replacement on insulin sensitivity in postmenopausal women. J Clin Endocrinol Metab. 1995;80:1783–1788. doi: 10.1210/jcem.80.6.7775623. [DOI] [PubMed] [Google Scholar]
- 25.Saglam K, Polat Z, Yilmaz MI, Gulec M, Akinci SB. Effects of postmenopausal hormone replacement therapy on insulin resistance. Endocrine. 2002;18(3):211–214. doi: 10.1385/ENDO:18:3:211. [DOI] [PubMed] [Google Scholar]
- 26.Duncan AC, Lyall H, Roberts RN, Petrie JR, Perera MJ, Monaghan S, et al. The effect of estradiol and a combined estradiol/progestagen preparation on insulin sensitivity in healthy postmenopausal women. J Clin Endocrinol Metab. 1999;84(7):2402–2407. doi: 10.1210/jcem.84.7.5836. [DOI] [PubMed] [Google Scholar]
- 27.Kimmerle R, Heinemann L, Heise T, Bender R, Weyer C, Hirschberger S, et al. Influence of continuous combined estradiol-norethisterone acetate preparations on insulin sensitivity in postmenopausal nondiabetic women. Menopause. 1999;6(1):36–42. [PubMed] [Google Scholar]
- 28.Ryan AS, Nicklas BJ, Berman DM. Hormone replacement therapy, insulin sensitivity, and abdominal obesity in postmenopausal women. Diabetes Care. 2002;25(1):127–133. doi: 10.2337/diacare.25.1.127. [DOI] [PubMed] [Google Scholar]
- 29.Van Pelt RE, Gozansky WS, Schwartz RS, Kohrt WM. Intravenous estrogens increase insulin clearance and action in postmenopausal women. Am J Physiol Endocrinol Metab. 2003;285(2):E311–317. doi: 10.1152/ajpendo.00490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Espeland MA, Hogan PE, Fineberg SE, Howard G, Schrott H, Waclawiw MA, et al. Effect of postmenopausal hormone therapy on glucose and insulin concentrations. PEPI Investigators. Postmenopausal Estrogen/Progestin Interventions. Diabetes Care. 1998;21(10):1589–1595. doi: 10.2337/diacare.21.10.1589. [DOI] [PubMed] [Google Scholar]
- 31.Kanaya AM, Herrington D, Vittinghoff E, Lin F, Grady D, Bittner V, et al. Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138(1):1–9. doi: 10.7326/0003-4819-138-1-200301070-00005. [DOI] [PubMed] [Google Scholar]
- 32.Cagnacci A, Soldani R, Carriero PL, Paoletti AM, Fioretti P, Melis GB. Effects of low doses of transdermal 17 beta-estradiol on carbohydrate metabolism in postmenopausal women. J Clin Endocrinol Metab. 1992;74(6):1396–1400. doi: 10.1210/jcem.74.6.1317387. [DOI] [PubMed] [Google Scholar]
- 33.Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cagnacci A, Paoletti AM, Zanni A, Arangino S, Ibba G, Orru M, et al. Raloxifene does not modify insulin sensitivity and glucose metabolism in postmenopausal women. J Clin Endocrinol Metab. 2002;87(9):4117–4121. doi: 10.1210/jc.2002-020120. [DOI] [PubMed] [Google Scholar]
- 35.Lee CC, Kasa-Vubu JZ, Supiano MA. Differential effects of raloxifene and estrogen on insulin sensitivity in postmenopausal women. J Am Geriatr Soc. 2003;51(5):683–688. doi: 10.1034/j.1600-0579.2003.00214.x. [DOI] [PubMed] [Google Scholar]
- 36.Shlipak MG, Chaput LA, Vittinghoff E, Lin F, Bittner V, Knopp RH, et al. Lipid changes on hormone therapy and coronary heart disease events in the Heart and Estrogen/progestin Replacement Study (HERS) Am Heart J. 2003;146(5):870–875. doi: 10.1016/S0002-8703(03)00412-5. [DOI] [PubMed] [Google Scholar]
- 37.Mackey RH, Kuller LH, Sutton-Tyrrell K, Evans RW, Holubkov R, Matthews KA. Hormone therapy, lipoprotein subclasses, and coronary calcification: the Healthy Women Study. Arch Intern Med. 2005;165(5):510–515. doi: 10.1001/archinte.165.5.510. [DOI] [PubMed] [Google Scholar]
- 38.Espeland MA, Marcovina SM, Miller V, Wood PD, Wasilauskas C, Sherwin R, et al. Effect of postmenopausal hormone therapy on lipoprotein(a) concentration. PEPI Investigators. Postmenopausal Estrogen/Progestin Interventions. Circulation. 1998;97(10):979–986. doi: 10.1161/01.cir.97.10.979. [DOI] [PubMed] [Google Scholar]
- 39.Shaw K, Gennat H, O’Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006;(4):CD003817. doi: 10.1002/14651858.CD003817.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katzel LI, Bleecker ER, Colman EG, Rogus EM, Sorkin JD, Goldberg AP. Effects of weight loss vs aerobic exercise training on risk factors for coronary disease in healthy, obese, middle-aged and older men. A randomized controlled trial. JAMA. 1995;274(24):1915–1921. doi: 10.1001/jama.1995.03530240025035. [DOI] [PubMed] [Google Scholar]