Abstract
Patients with compression of the celiac axis by the median arcuate ligament may develop aneurysms in the pancreaticoduodenal arcades. We experienced two cases of ruptured pancreaticoduodenal artery aneurysm associated with this condition. Both patients presented with abdominal pain and shock, and abdominal contrast-enhanced computed tomography showed retroperitoneal hematoma and compression of the celiac axis by the median arcuate ligament. Both patients were successfully treated by coil embolization. Patients with celiac axis compression or stenosis may develop recurrent aneurysms unless revascularization of the celiac axis is performed. Long-term follow-up is required because aneurysms may develop after 10 years or longer.
Keywords: celiac axis stenosis, median arcuate ligament, pancreaticoduodenal artery aneurysm
Introduction
The median arcuate ligament (MAL) is a fibrous arch connecting the medial borders of the diaphragmatic crura on either side of the aortic hiatus. Compression of the celiac axis by the MAL may cause foregut ischemia. Such ischemia may result in the rare condition known as median arcuate ligament syndrome (MALS), which is characterized by weight loss, postprandial abdominal pain, nausea, and vomiting.1) Strategies for the treatment of MALS have focused on restoration of the blood flow in the celiac artery. Decompression of the celiac artery by division of the MAL relieves the symptoms in some patients, and stenting or bypass grafting may be considered in cases with insufficient celiac artery flow after MAL division.2)
Compression of the celiac axis by the MAL and the resultant foregut ischemia commonly lead to the development of collateral circulation from branches of the superior mesenteric artery (SMA).3) The network of arterial arcades between the superior and inferior portions of the anterior and posterior pancreaticoduodenal arteries (PDAs) provides a connection between the SMA system and the celiac artery system,3) and increased flow through these vessels may result in the formation of PDA aneurysms.3) Rupture of a PDA aneurysm can cause potentially fatal bleeding into the retroperitoneal space, abdominal cavity, or gastrointestinal tract.4) Ruptured aneurysms can be effectively treated by immediate embolization, which has a lower mortality rate than surgical treatment.4)
We report 2 patients who presented with abdominal pain and shock due to ruptured PDA aneurysms associated with compression of the celiac axis by the MAL. In one patient, the anterior PDA was larger than the posterior PDA and accounted for a significant portion of the collateral flow. In the other patient, there was equal enlargement of the anterior and posterior PDAs. In both patients, urgent coil embolization of the PDA supplying the ruptured aneurysm via the SMA resulted in successful treatment without complications. We also review recently reported cases of ruptured PDA aneurysms associated with compression of the celiac axis by the MAL.
Case Report
Case 1
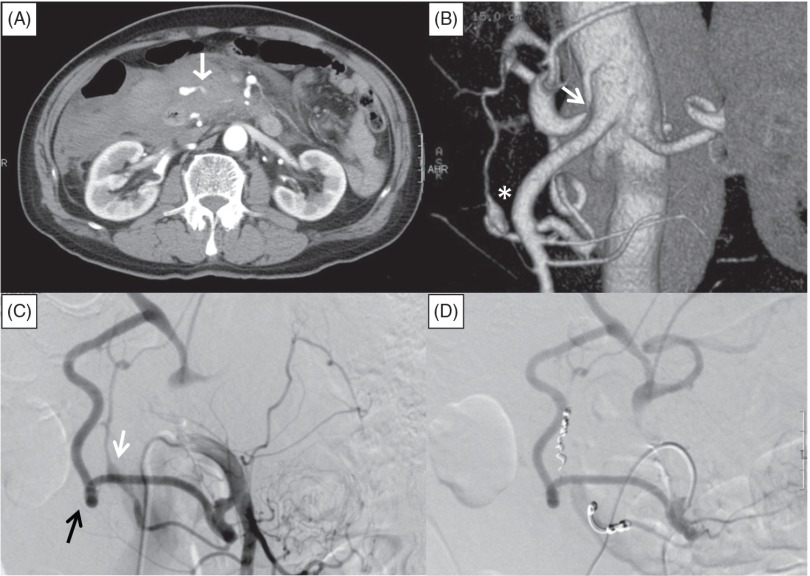
An 80-year-old man was admitted to the emergency department with a 24-hour history of abdominal pain and vomiting. He was a non-smoker and a light drinker, and reported no remarkable medical history except for lumbar intervertebral disc herniation. He was not taking any medications. At admission, his systolic blood pressure was 88 mmHg, diastolic blood pressure was undetectable, heart rate was 114 beats/min, respiratory rate was 30 breaths/min, hemoglobin level was 9.8 g/dL, and hematocrit was 30.3%. He was sweaty with cold extremities. Abdominal palpation revealed supraumbilical tenderness. His vomitus did not contain blood, suggesting that he was not actively bleeding from the upper gastrointestinal tract. After intravenous infusion of 1 L of crystalloid fluid, his blood pressure increased to 110/90 mmHg. Abdominal contrast-enhanced computed tomography (CT) showed retroperitoneal hematoma with extravasation from an artery adjacent to the dorsal portion of the pancreas (Fig. 1A). Reconstructed sagittal CT images showed compression of the celiac axis by the MAL. Three-dimensional CT images showed focal narrowing of the celiac axis and an aneurysm in the pancreaticoduodenal arcades (Fig. 1B). Selective catheter angiography of the SMA showed retrograde flow from the SMA to the celiac artery via the pancreaticoduodenal arcades, and extravasation of contrast medium from a ruptured aneurysm (10 mm in diameter) of the posterior inferior PDA (PIPDA) (Fig. 1C). The anterior PDA was obviously larger than the posterior PDA, and provided the majority of the collateral flow. A 4-F catheter (Angiomaster; Terumo Clinical Supply, Tokyo, Japan) was advanced into the SMA and a microcatheter (Sniper 2-µ7; Terumo, Japan) was advanced through the PIPDA into the posterior superior PDA (PSPDA). Embolization was performed with microcoils (Tornado; Cook Medical, Bloomington, Indiana, USA) placed in the PSPDA and PIPDA (Fig. 1D). Postprocedural CT showed no flow into the aneurysm. The patient had no periprocedural complications and was discharged on day 13. At the 6-month follow-up visit, he did not complain of any abdominal symptoms and there was no evidence of aneurysm recurrence. We intend to perform yearly abdominal contrast-enhanced CT examinations to screen for further aneurysm formation.
Fig. 1.
Abdominal contrast-enhanced computed tomography (CT) and angiography of the superior mesenteric artery (SMA) in Case 1. (A) Retroperitoneal hematoma with extravasation from an artery (white arrow) adjacent to the dorsal side of the pancreatic head. (B) Three-dimensional CT image showing luminal stenosis of the celiac axis (white arrow) and an aneurysm of the pancreaticoduodenal artery (PDA) (asterisk). (C) Selective catheter angiography of the SMA showing retrograde flow from the SMA to the common hepatic artery via the larger anterior PDA (black arrow) and the smaller posterior PDA with an aneurysm (white arrow). (D) SMA angiography after embolization, showing no flow into the posterior PDA and the aneurysm.
Case 2
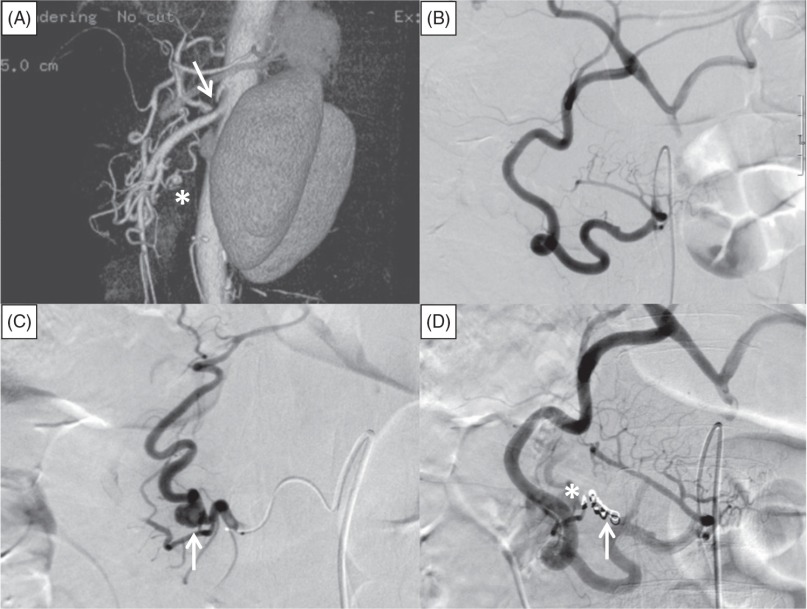
A 58-year-old man with a history of hypertension was admitted to the emergency department an hour after the sudden onset of severe epigastric pain with nausea and vomiting. He was a non-smoker and non-drinker, and had no past medical history except for hypertension. He had taken losartan (25 mg/day) for 8 years. On admission, his blood pressure was 84/50 mmHg, heart rate was 108 beats/min, respiratory rate was 26 breaths/min, white blood cell count was 15400/µL, hemoglobin level was 10.8 g/dL, and hematocrit was 33.6%. Abdominal palpation revealed epigastric tenderness, but no signs of peritoneal irritation. He vomited several times soon after admission, but his vomitus did not contain blood, and no melena was observed. Abdominal contrast-enhanced CT showed retroperitoneal hematoma around the pancreatic head and body, but the source of bleeding was not evident. Reconstructed CT images showed compression of the celiac axis from above by the MAL, and three-dimensional CT images showed stenosis of the celiac axis and a small aneurysm (12 mm in diameter) in the pancreaticoduodenal arcades (Fig. 2A). Selective angiography of the SMA using a 4-F catheter (Angiomaster; Terumo, Japan) showed retrograde flow from the SMA to the celiac artery via the pancreaticoduodenal arcades. Superselective angiography using a microcatheter (Sniper 2-µ7; Terumo, Japan) showed no aneurysm in the anterior PDA (Fig. 2B) and a 12-mm diameter aneurysm with slight extravasation in the PIPDA (Fig. 2C). The anterior and posterior PDAs were equal in size. We tried to perform embolization by placing microcoils (Tornado; Cook Medical, USA) in the PSPDA via the PIPDA, but were unable to advance the microcatheter across the aneurysm because of a bend in the vessel. Therefore, microcoils were first placed in the proximal PIPDA to obstruct the afferent flow of the aneurysm, and additional microcoils were placed in the branch of the anterior PDA that fed the aneurysm (Fig. 2D). Postprocedural CT showed no flow into the aneurysm. There were no further signs of bleeding from the aneurysm during admission. The patient had no periprocedural complications and was discharged on day 14. At the 6-month follow-up visit, he did not report any abdominal symptoms and there was no evidence of aneurysm recurrence on abdominal contrast-enhanced CT. We intend to perform yearly abdominal contrast-enhanced CT examinations to screen for further aneurysm formation.
Fig. 2.
Abdominal contrast-enhanced computed tomography (CT) and angiography of the superior mesenteric artery (SMA) in Case 2. (A) Three-dimensional CT image showing stenosis of the celiac axis due to compression by the median arcuate ligament (white arrow), and an aneurysm in the pancreaticoduodenal arcades (asterisk). (B) Superselective angiography of the anterior pancreaticoduodenal artery (PDA) did not show any aneurysms. (C) Superselective angiography of the posterior PDA showed an aneurysm measuring 12 mm in diameter (white arrow) with slight extravasation. (D) SMA angiography after embolization did not show the aneurysm. Microcoils are visible in the proximal portion of the posterior inferior PDA (white arrow) and in the branch of the anterior PDA (asterisk).
Discussion
Aneurysms of the PDA are rare, accounting for <2% of all visceral artery aneurysms, and the symptoms can vary from none to catastrophic rupture.3,4) Factors associated with the formation of PDA aneurysms include atherosclerosis, local infection, trauma, pancreatitis, fibromuscular dysplasia, congenital anomalies, and compression of the celiac axis by the MAL.3,5) The reported incidence of celiac axis stenosis ranges from 12.5% to 24.5% in Western populations.5) Varying degrees of celiac axis compression by the MAL are observed in 13%–50% of abdominal arteriograms, and severe stenosis of the celiac axis due to compression by the MAL is observed in 1% of arteriograms.1,5) However, the prevalence of celiac axis stenosis due to compression by the MAL is unclear, especially in asymptomatic cases. We reviewed the PubMed database from 2000 to 2013, and found eight reports of Japanese patients with ruptured PDA aneurysms associated with compression of the celiac axis by the MAL (Table 1).4,6–10) The mean age of these 8 patients was 56.9 years, and five of them were male. Most of the aneurysms were in the inferior PDA, and the mean aneurysm size was 1.5 cm. At least four of the patients presented with hemorrhagic shock.4,6,7,10) All patients were successfully treated by transarterial coil embolization. Among the 10 reported UK patients with ruptured PDA aneurysms associated with compression of the celiac axis by the MAL during the same time period, the mean age was 39.5 years, 3 patients were male, and the mean aneurysm size was 2.8 cm. UK patients were more likely to be female, were younger, and had a larger aneurysm size than Japanese patients.3) These findings may reflect racial differences.

It is interesting that 86% of reported patients with MALS who underwent surgical division of the MAL but did not have PDA aneurysms were female, and their mean age was 44.6 years.2) Our patients were both elderly males, and our review of reported Japanese cases suggests that males may have a higher risk of aneurysm formation or rupture secondary to compression of the celiac axis by the MAL than females. In addition, the higher mean age of patients with ruptured PDA aneurysms compared with patients treated for MALS without PDA aneurysms may reflect an age-related etiologic factor such as atherosclerosis, which was reported to be associated with the development of PDA aneurysms.3,5) Strangely, chronic symptoms characteristic of MALS before PDA aneurysm rupture were reported in only one of the reviewed Japanese cases.7) Separately from the reviewed Japanese patients, unruptured PDA aneurysms were discovered incidentally on abdominal CT examinations in four Japanese cases, and these patients did not have abdominal symptoms.5,8) Our 2 patients were also asymptomatic before rupture of the aneurysms. This suggests that the symptoms associated with MALS may sometimes be caused by factors other than organ ischemia. In patients with MALS, resection of the periarterial neural tissues comprising the celiac plexus or ganglion results in better symptom relief than vascular reconstruction of the celiac axis alone,2) suggesting involvement of neural factors in the symptoms of MALS.2) A recent review reported 10 patients with PDA aneurysms associated with stenosis or occlusion of the celiac axis due to compression by the MAL, and none of these patients presented with classic symptoms of MALS.3) The reason for absence of MALS symptoms in some patients with foregut ischemia due to celiac axis stenosis remains unclear, and further investigation is required.
Treatment of PDA aneurysms by embolization has a lower risk of complications and mortality than surgical treatment.4) The size of the PDA aneurysm is not associated with the risk of rupture, and the majority of ruptured aneurysms are <10 mm in diameter.3) Elective coil embolization can therefore be performed in patients with unruptured PDA aneurysms.4,5,8) However, the long-term prognosis after embolization is still unclear. In patients with celiac axis stenosis and PDA aneurysms, coil embolization without vascular reconstruction may lead to recurrence of PDA aneurysms.4) There are currently no reported cases of recurrent PDA aneurysm formation associated with celiac axis stenosis or occlusion after coil embolization. Flood and Nicholson studied 14 patients with inferior PDA aneurysms associated with celiac axis stenosis who mainly underwent embolization or combination therapy (embolization and stenting of the celiac axis).3) No recurrence was detected in any of these patients, but the longest follow-up period was 4 years. They also reviewed 49 reported patients who were treated for PDA aneurysms associated with celiac axis stenosis. Although 40 of these patients did not undergo revascularization of the celiac axis, no recurrence of aneurysms was reported, but the longest follow-up period was 3 years. However, the mean age of the Japanese patients reviewed in this study (56.9 years) is higher than that of reported MALS patients without aneurysms (44.6 years). If the difference in age between these two populations (approximately 10 years) reflects the time for formation of aneurysms, the previously reported follow-up periods may be too short to detect recurrence. Although there is currently no consensus regarding optimal follow-up of patients treated for PDA aneurysms, careful follow-up for more than 10 years may enable early detection of recurrent PDA aneurysms. Aggressive treatment to prevent further aneurysm formation, such as revascularization or decompression of the celiac axis, may also be beneficial. Decompression of the celiac axis by surgical division of the MAL may be a useful treatment option, but luminal stenosis may persist after surgery.1) Primary balloon angioplasty and stenting without prior division of the MAL may result in stent slippage or failure.1) In patients with PDA aneurysms associated with celiac axis stenosis due to compression by the MAL, combined treatment by surgical decompression and endovascular angioplasty/stenting may therefore be optimal. Surgical decompression by vascular reconstruction with bypass grafting is an alternative to endovascular treatment, especially when selective catheterization of the celiac artery is difficult.1,3)
In conclusion, we experienced two patients with ruptured PDA aneurysms associated with compression of the celiac axis by the MAL. Japanese patients with ruptured PDA aneurysms are older and more likely to be male than reported patients requiring treatment for MALS who do not have aneurysms. Coil embolization is a useful treatment for ruptured PDA aneurysms, but patients may be at risk of further aneurysm formation because of the persistently increased blood flow secondary to celiac axis stenosis. Further investigation including longer-term follow-up is needed to determine the need for additional treatment such as decompression and revascularization of the celiac axis in patients treated for PDA aneurysms associated with compression of the celiac axis by the MAL.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- Delis KT, Gloviczki P, Altuwaijri M, et al. Median arcuate ligament syndrome: open celiac artery reconstruction and ligament division after endovascular failure. J Vasc Surg 2007; 46: 799-802 [DOI] [PubMed] [Google Scholar]
- Tulloch AW, Jimenez JC, Lawrence PF, et al. Laparoscopic versus open celiac ganglionectomy in patients with median arcuate ligament syndrome. J Vasc Surg 2010; 52: 1283-9 [DOI] [PubMed] [Google Scholar]
- Flood K, Nicholson AA. Inferior pancreaticoduodenal artery aneurysms associated with occlusive lesions of the celiac axis: diagnosis, treatment options, outcomes, and review of the literature. Cardiovasc Intervent Radiol 2013; 36: 578-87 [DOI] [PubMed] [Google Scholar]
- Murata S, Tajima H, Fukunaga T, et al. Management of pancreaticoduodenal artery aneurysms: results of superselective transcatheter embolization. AJR Am J Roentgenol 2006; 187: W290-8 [DOI] [PubMed] [Google Scholar]
- Ikeda O, Tamura Y, Nakasone Y, et al. Coil embolization of pancreaticoduodenal artery aneurysms associated with celiac artery stenosis: report of three cases. Cardiovasc Intervent Radiol 2007; 30: 504-7 [DOI] [PubMed] [Google Scholar]
- Ogino H, Sato Y, Banno T, et al. Embolization in a patient with ruptured anterior inferior pancreaticoduodenal arterial aneurysm with median arcuate ligament syndrome. Cardiovasc Intervent Radiol 2002; 25: 318-9 [DOI] [PubMed] [Google Scholar]
- Akatsu T, Hayashi S, Yamane T, et al. Emergency embolization of a ruptured pancreaticoduodenal artery aneurysm associated with the median arcuate ligament syndrome. J Gastroenterol Hepatol 2004; 19: 482-3 [DOI] [PubMed] [Google Scholar]
- Sugiyama K, Takehara Y. Analysis of five cases of splanchnic artery aneurysm associated with coeliac artery stenosis due to compression by the median arcuate ligament. Clin Radiol 2007; 62: 688-93 [DOI] [PubMed] [Google Scholar]
- Watanabe A, Kohtake H, Furui S, et al. Celiac artery dissection seen with ruptured pancreaticoduodenal arcade aneurysms in two cases of celiac artery stenosis from compression by median arcuate ligament. J Vasc Surg 2012; 56: 1114-8 [DOI] [PubMed] [Google Scholar]
- Mastumura Y, Nakada TA, Kobe Y, et al. Median arcuate ligament syndrome presenting as hemorrhagic shock. Am J Emerg Med 2013; 31: 1152.e1-4 [DOI] [PubMed] [Google Scholar]