Figure 2.
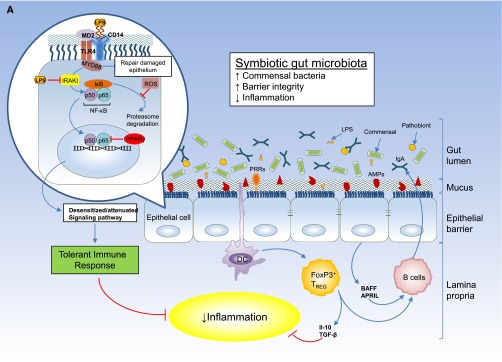
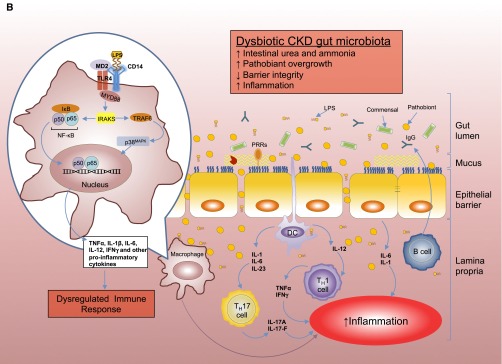
(A) Intestinal epithelial barrier and inflammatory responses in symbiotic and dysbiotic gut microbiota. A symbiotic gut microbiota leads to development of a functional barrier, with normal amounts of mucus, pattern recognition receptors (PRRs), antimicrobial peptides (AMPs), and secreted IgA, which in turn contain the microbiota in the intestinal lumen and away from the intestinal epithelial cells. As a result, the intestinal immune system becomes largely tolerant to the resident commensals. Similar to immune cells, the signaling cascades that occur downstream of TLRs (enlarged on the left) are used by epithelial cells to detect microbes through PRRs, such as the TLR4. Briefly, upon LPS ligation, the MYD88 is recruited, which activates the NF-κB pathway and leads to production of antimicrobial proteins and proinflammatory cytokines. In a symbiotic gut, epithelial cells are desensitized by continuous exposure to LPS168 or are attenuated by (1) LPS-mediated downregulation of the IL-1 receptor–associated kinase 1 (IRAK1), which is the proximal activator of the NF-κB cascade;168 (2) LPS-mediated induction of peroxisome proliferator-activated receptor-γ (PPArγ), which can divert NF-κB from the nucleus;169 or (3) commensal bacteria-derived reactive oxygen species (ROS)–mediated inhibition of polyubiquitylation and degradation of the aortic inhibitor of κB.170 (T bars indicate the checkpoints that are controlled by the microbiota.) Exposure to LPS induces epithelial cells to secrete TGF-β, B-cell–activating factor of the TNF family (BAFF), and a proliferation-inducing ligand (APRIL), all promoting the development of tolerogenic immune cell responses to the microbiota. CD103+ dendritic cells (DCs) support the development of regulatory T (Treg) cells secreting IL-10 and TGF-β, and together they stimulate the production of commensal-specific IgA.171 (B) Increased intestinal concentration of uremic toxins associated with the progression of CKD leads to microbial dysbiosis and overgrowth of pathobionts. Pathobiont overgrowth leads to the loss of barrier integrity and the breach in the epithelia barrier. Translocation of bacteria and bacterial components triggers the intestinal immune system to direct a potentially harmful proinflammatory response to clear invading bacteria by secreting IL-1 and -6 from intestinal epithelial cells, promoting a TH1 and TH17 response by DCs and macrophages and producing higher levels of commensal-specific IgG by B cells. In this context, LPS binding to its receptor complex on macrophages (enlarged on the left) results in enhanced production of inflammatory cytokines including IFN-β, IFN-γ, IL-1β, IL-6, TNFα, and IL-12, the production of which has been shown to require activation of p38MAPK.172 Subclinical endotoxemia is a potential cause of inflammation in CKD.90–92 Dysregulated immune response and chronic production of proinflammatory cytokines lead to systemic inflammation, which could further accelerate the progression of CKD and development of cardiovascular disease. IκB, inhibitor of NF-κB.
