Abstract
Introduction:
Bone disease and short stature are frequent clinical features of patients with beta-thalassaemia major. Dysfunction of the GH-IGF-1 axis has been described in many thalassemics children and adolescents with short stature and reduced growth velocity. Assessment of the GH-IGF-1 axis in short adults with TM after attainment of final height may be required to select those who are candidates for replacement therapy and to prevent the development of bone disease. The aim of our study was to investigate GH secretion in adult thalassemic patients in relation to their bone mineral density (BMD) and serum ferritin concentrations.
Materials and Methods:
We performed clonidine stimulation test in 30 thalassemic patients (18 males, 12 females) with a mean age of 31.5± 7.2 years. The cut-off level for GH response was set at 7ug/l, according to the literature. Serum ferritin, IGF-I, liver enzymes, alkaline phosphatase (ALP) and type 1 Collagen Carboxy Telopeptide (CCT1) were also determined.
Results:
We diagnosed GH deficiency (GHD) in 12 patients (40%) and IGF-I deficiency (IGF-I SDS <-2) was diagnosed in 20 patients (67%). Adult patients with TM had significantly decreased IGF-I concentrations and bone mineral density (BMD) at the femur neck and lumbar spine compared to normal controls. Thalassemic patients with GHD and IGF-I deficiency had significantly lower BMD T score at the lumbar spine compared to patients with normal GH and IGF-I levels. Thalassemic patients had higher serum CCT1 concentrations compared to normal controls. Peak GH levels were correlated significantly with IGF- I concentrations and IGF-I levels were correlated significantly with the height SDS (HtSDS) of thalassemic patients. Neither GH peak nor IGF-I concentrations were correlated to serum ferritin concentrations.
Conclusions:
We conclude that GH status should be tested in adult thalassemic patients especially those with short stature and/or decreased BMD. Clonidine test appears to be effective and safe in adults with TM. If the diagnosis of adult GHD is established, GH treatment may be considered for possible improvement of bone mineral density and heart function in patients with TM.
Keywords: Bone mineral density, ferritin, GH deficiency GHD, insulin like growth factor –I, growth, growth hormone, prevalence, thalassemia
INTRODUCTION
Adults over the age of 18 years with thalassemia major with repeated blood transfusion suffer from high iron overload in several organs (mainly heart and liver) and endocrine glands.[1,2]
Variable prevalence of growth hormone deficiency (GHD) and/or insulin-like growth factor-I (IGF-I) deficiency, both in children and adults, are reported in different centers caring for thalassemic patients. Recent studies have demonstrated the necessity to evaluate adult patients affected by TM to establish the presence of GH-IGF-I alteration which could be relevant in the pathogenesis of bone disease and cardiac dysfunction frequently present in this haemoglobinopathy.[3,4,5,6,7]
Adult patients with TM have symptoms and signs that are indistinguishable from those of GHD. These include lack of positive wellbeing, depressed mood, feelings of social isolation, decreased energy and an overall poorer quality of life when compared with controls. Their bone mineral density (BMD) is also reduced, resulting in increase in bone fracture rate.[8,9,10] The combined growth hormone releasing (GHRH) + arginine test is expensive and not available in many centres whereas, glucagon stimulation test requires 4 h for adequate sampling.[11]
Although clonidine stimulation test is used frequently in children and proved to be easy, effective, safe and relatively cheap, it is seldom used in adults.[12]
The relationships between bone density and several clinical characteristics or hematological markers have been described and many factors contributing to demineralization have been identified; among them endocrine complications including GH and IGF-I deficiency seem to play an important role.[13,14]
The aim of this study was to evaluate GH response to clonidine and measure serum IGF-I concentrations in relation to auxological and bone mineral density in 30 polytransfused adult patients with thalassemia major (TM).
MATERIALS AND METHODS
Thirty (18 males and 12 females, mean age of 31.5 ± 7.2 years) randomly selected euthyroid patients with beta thalassemia with full pubertal development and normal glucose tolerance were studied. They were regularly transfused, since early childhood and underwent chelation therapy using desferrioxamine which was replaced by deferasirox for the last 4 to 5 years with moderate compliance to oral chelation in most of the patients (22 patients had ferritin level >1500 and <2500 ng/ml).
BMD of lumbar spine and right femoral neck were measured by dual-energy X-ray absorptiometry (DEXA) scan using a calibrated dual energy X-ray absorption method. Osteoporosis as per WHO criteria (T score of less than -1.0 being defined as osteopenic and a T score of less than -2.5 being referred as osteoporotic). All patients were evaluated biochemically by checking their serum calcium, phosphorus, bone-specific-alkaline phosphatase (ALP) and type 1 Collagen Carboxy Telopeptide (CCT1), using enzyme-linked immunosorbent assay (ELISA). All patients had normal thyroid function and none of the patients had hypoparathyroidism or adrenal insufficiency. None of the patients had history of fracture. All patients with delayed puberty (5 males and 3 females) were on sex steroid therapy. All patients were taking vitamin D3 800 U/day orally for the past 3 years. None of the patients was receiving calcium. All patients were vaccinated against hepatitis B virus and all were negative for hepatitis screening (HVB and HVC). Growth hormone (GH) secretion was investigated using standard clonidine provocation test. Their circulating insulin-like growth factors (IGF) and liver enzymes (alanine transferase (ALT) and aspartate transferase (AST)) concentrations were measured. The mean serum ferritin concentrations in the previous 5 years was calculated and recorded.
At the time of the study their serum ferritin levels were 2488 ± 1557 ng/ml and Hb levels were 10.7 ± 1.2 g/dl. Hepatic enzymes ALT and AST were within the normal range (49 ± 15 IU and 47 ± 21 IU respectively).
GH concentrations were measured by immunoradiometric assay (IRMA) using commercially available kits. The sensitivity of the assay was 0.2 mg/L. The intra- and interassay coefficients of variation (CVs) were 4.5% and 7.9%, respectively. The cut-off level for GH response was set at 7 microgram/l, according to the literature. Plasma IGF-I was measured by IRMA after ethanol extraction using an ultrasensitive chemi-luminescence assay (sensitivity 0.002 microgram/l). The inter-assay CVs were 8.2%, 1.5% and 3.7% for low, medium and high points of the standard curve. IGF-I levels were transformed to age-dependent IGF-I SDS according to Brabant et al.[15] Type 1 Collagen Carboxy Telopeptide (CCT1) was analyzed by a radioimmunoassay (RIA) with polyclonal rabbit antibodies specific for human ICTP. Intra-assay and interassay variations were less than 6% and 7%, respectively.
Normative data for serum IGF-I were obtained from 50 subjects aged 20-40 years. Bone mineral density was measured using dual-energy X-ray absorptiometry. This control group was age, sex and BMI-matched [Table 1]. Informed consents have been obtained from all patients and controls and the Ethical Committee of Hamad Medical Center (HMC) approved the study.
Table 1.
Clinical and clinical and biochemical data of patients and controls

Regression analysis was performed to correlate GH peak after clonidine, IGF-I and serum ferritin concentrations with bone mineral density T- score at the lumbar spine and femoral neck. Student t test was used to compare variables between the groups when data were normally distributed and Wilcoxon test when the data were not normally distributed. Data are reported as the mean +/- SD. The limit of significance was set at 5%.
RESULTS
The auxological and biochemical data of the 30 adults with TM compared to 50 normal age-matched adults are presented in Table 1. Patients with TM were significantly shorter (HtSDS=-2.05+/- 0.4) than controls (HtSDS=-0.4+/- 0.3)) with markedly lower IGF-I and higher Type 1 collagen telopeptide concentrations compared to age matched controls (173+/- 65 ng/ml versus 339+/- 102 ng/ml and 1798+/- 562 pg/mL versus 408+/- 207 pg/mL respectively).
We diagnosed GH deficiency (GHD) in 12 adult patients with TM (40%). No side effects were noted in any patient after clonidine apart from mild drowsiness or sleepiness in 5 patients that disappeared after 4 to 6 h. None of the patients had significant hypotension. There were no significant differences between the GHD and GH sufficient (GHS) groups regarding the value of mean 5 year serum ferritin concentration and liver enzymes. The group affected by GHD were significantly shorter and had significantly lower IGF-I concentrations versus the GHS group. The BMD T score measured at the lumbar spine were significantly lower in the GHD group (P = 0.042) [Table 2].
Table 2.
Comparison between GH deficient versus GH sufficient thalassemic patients
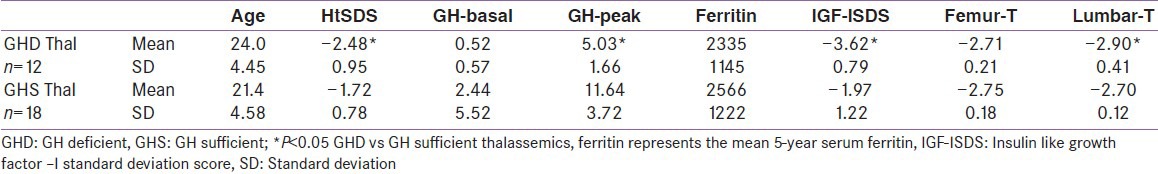
IGF-I deficiency (IGF-I SDS <-2) was diagnosed in 20 patients (67%), 12 of them had GHD. There were no significant differences between the IGF-I deficient and sufficient groups regarding the value of serum ferritin and liver enzymes. The IGF-I deficient group were significantly shorter and had lower peak GH concentrations versus the group with normal IGF-I level. The BMD T score measured at the lumbar spine were significantly lower in the GHD group (P = 0.029) and IGF-I deficient (P = 0.042) groups versus those with normal GH and IGF-I secretion [Tables 2 and 3]. Lumbar and femur neck osteoporosis was demonstrated in all patients (males and females) with and without GH-IGF-I abnormalities (BMD <-2.5 SD).
Table 3.
Comparison between IGF-I deficient and sufficient thalassemic patients
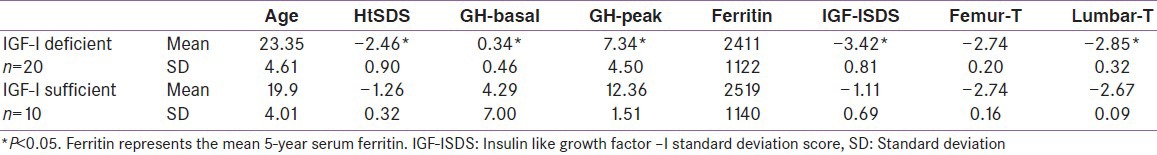
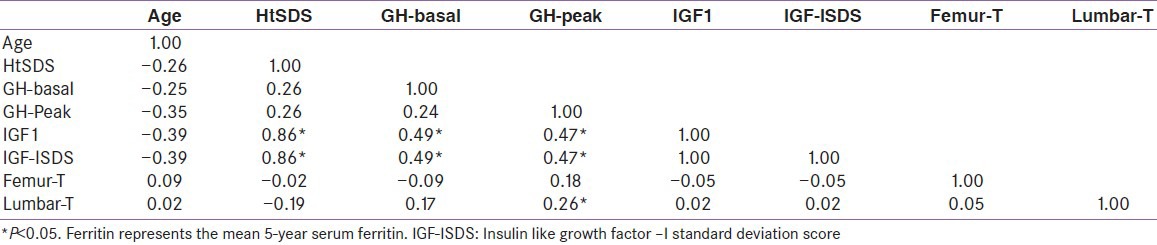
HtSDS was significantly correlated with IGF-I concentration [Figure 1]. There was a significant correlation between IGF-I SDS and GH peak concentrations (P = 0.0001). Lumbar T-score values were significantly correlated with peak GH concentrations (r = 0.26, P = 0.023) [Figure 2]. Femoral and lumbar T-score vales were not correlated significantly with the IGF-I SDS [Table 4]. No significant correlation was detected between serum ferritin concentrations and GH peak, IGF-1 or T- score in the whole group of patients. The average hemoglobin concentrations (before and after transfusion) for the last 5 years did not correlate with the lumbar and femoral BMI T scores (r = 0.02), or IGF-I SDS (r = 0.043).
Figure 1.
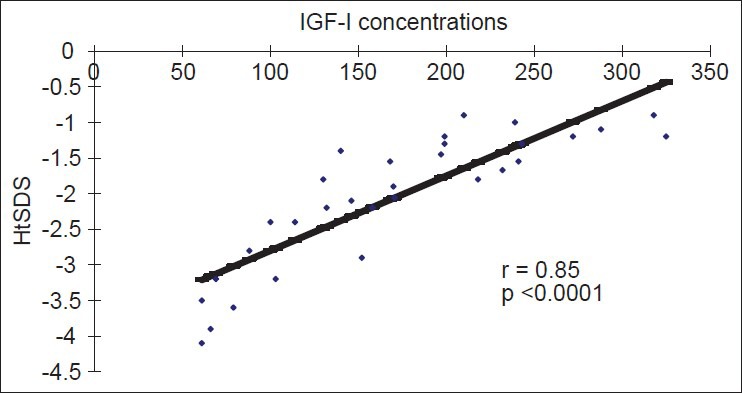
Regression of HtSDS on IGF-I Concentrations in adults with thalassemia
Figure 2.

Regression of peak growth hormone on bone mineral density T score of Lumbar vertebrae
Table 4.
Correlations between variable in adult thalassemic patients
DISCUSSION
In this study, the prevalence of GHD (40%) and IGF-I deficiency (67%) in 30 adults with TM was high. Similar high prevalence of GH deficiency was reported by others.[3,4,5,6,7] Peak GH to provocation by clonidine correlated well with IGF-I concentrations and therefore seems to be effective. The absence of significant side-effects in 30 adults with TM suggests its safe use in adults.
Osteopenia, reduced lean mass, altered cardiac structure, unfavorable glucose metabolism, reduced exercise capacity and reduced quality of life are reported in adults with GHD as well as those with TM.[16,17,18,19]
Growth hormone deficiency (GHD) in adults with TM can explain in part the low IGF-I synthesis. Our thalassemic patients with GHD had significantly lower IGF-I concentrations versus those with normal GH secretion. Soliman et al.,[20] showed that the IGF-I levels were significantly higher in thalassemic children with normal GH secretion versus those with GHD, after 12 years of age. However, in this study as well as in other cross-sectional studies, patients with TM at different ages who have normal GH secretion (GHS) still have high prevalence of low serum IGF-I.[21,22,23] A longitudinal study in thalassemic children followed the age-related changes in serum IGF-I concentrations in thalassemic subjects (n = 21), compared to normal standards for age and sex, showed significantly lower IGF-I concentrations starting from early childhood to 18 years of age. Thalassemic children with GHD did not show any peak of IGF-I till 18 years of age, whereas those with normal GH secretion achieved markedly attenuated and late peak of IGF-I level versus normal males.[21,22,23] IGF-I deficiency (IGF-I SDS <-2) was diagnosed in 20 patients (67%), 12 of them had GHD and a significant correlation was detected between IGF-I SDS and GH peak concentrations. These findings suggest that GH secretory status remains a major regulator of IGF-I production in adult patients with major thalassaemia.
In thalassemic adults with normal GH secretion, the low basal level of IGF-I compared to normal controls, suggested either a state of decreased GH sensitivity (secondary to hepatic iron overload) and/or GH neuro-secretory dysfunction.[20,21,22,23]
Excessive iron deposition in the pituitary and liver appear to be the major etiology for GH and/or IGF-I deficiency. Single injection of iron nano particles in mice induced inflammation with significantly increased levels of pro-inflammatory cytokines (IL-1, TNF-alpha, and IL-6).[24] Recent data demonstrated increased circulating levels of IL-1α, TNF-α and IL-6 in patients with BTM which may explain the gradual and progressive deterioration of organs including the pituitary (GH secretion) liver (IGF-I secretion) and growth plate in children with TM.[25] An important mechanism for attenuation of GH signaling involves members of a family of cytokine-inducible genes identified by the acronym SOCS (suppressors of cytokine signaling). Available evidence indicates that the several SOCS family members can inhibit JAK- mediated.[26] In support of the deleterious effect of iron toxicity on IGF-I secretion, serum ferritin was negatively correlated with IGF-I concentrations in children with TM.[20,21,22] Our adult TM patients had prolonged (5 years or more) suboptimal level of iron chelation (ferrtin >1500 ng/ml), even after use of oral chelation therapy, can explain their pituitary and hepatic iron overload. The presence of chronic intermittent anemia in these patients may significantly add to the cellular dysfunction in the pituitary and hepatic cells (GH-IGF-I secretion). In this study serum ferritin levels was not correlated with GH or IGF-I levels or BMD T. In support with our results, other studies scarce or total lack of correlation has been shown between serum ferritin and hepatic iron measured by MRI with many discrepancies recorded in several patients.[27,28]
Chronic hemolysis and chronic anemia (hypoxia) constitute a considerable stress to a thalassemic patient. Patho-physiological stress may lead to GH resistance through cytokine-mediated induction of SOCS family members.[29] The association between increased circulating levels of pro-resorptive cytokines and an altered bone turnover in TM patients suggested their involvement in the pathogenesis of TM-osteoporosis.[30] Studies using transgenic murine models have examined the systemic effects of cytokines on the GH/IGF-1 axis. High levels of circulating IL-6 reduced growth rate. The growth defect is completely abolished by neutralization of IL-6. In mice increased cytokines was associated with reduced circulating IGF-1 levels, while GH production remains unaltered.[31,32,33,34] This mirrors the observations in thalassemic patients with GHS,[20,21,22,23,35] and goes with the finding that the effect on IGF-1 levels is not totally mediated via an effect on GH production.
Osteopenia and osteoporosis represent prominent causes of morbidity in patients of both genders with thalassaemia. All our eugonadal euthyroid thalassemic patients with normal glucose tolerance had osteoporosis detected at the lumbar spine and femoral neck. The presence of osteopenia and osteoporosis in well-treated thalassaemics has been described in different studies with high to very high prevalence.[13,14,36] Bone remodeling is a spatially coordinated lifelong process whereby old bone is absorbed by osteoclasts and replaced by bone-forming osteoblasts. The catabolic effects of Receptor activator of nuclear factor-κB ligand (RANKL) are prevented by osteoprotegerin (OPG) that binds RANKL and thereby prevents activation of its single cognate receptor called RANK.
Important factors implicated in reduction of bone mass in TM include: Delayed sexual maturation, growth hormone (GH) and insulin growth factor-(IGF)-1 deficiency, parathyroid gland dysfunction, diabetes, hypothyroidism, ineffective hemopoiesis with progressive marrow expansion, direct iron toxicity on osteoblasts, as well as toxic effect of chelating therapy have been indicated as possible etiological factors.[36,37,38] Our thalassemic patients had high prevalence of GH and IGF-I deficiency.
Reduced BMD has been widely reported in hypopituitary patients and patients with isolated GHD as well as those with congenital GH insensitivity were osteopenic compared to controls.[39,40,41,42,43,44] The pathogenesis of the reduced bone mineral content and density in patients with childhood onset GHD, including thalassemic patients with GHD, is probably the lack of attainment of bone mass during adolescence,[20,45,46,47] whereas in patients with adult-onset GHD it is less clear. Prolonged treatment with GH resulted in increased BMD.[45,46,47,48] With this study in addition with several previous reports, bone loss occurs preferentially in lumbar spine. This can be explained by the occurrence of accelerated hematopoiesis with progressive bone marrow expansion during the periods of anemia that affects trabecular bone to a greater extent than cortical bone.
In this study, both groups of thalassemic patients with and without GH-IGF-I deficiency had significant reduction of BMD at the femur neck and lumbar spine (T score < 2.5). However; the BMD at the lumbar spine was significantly lower in patients with GH-IGF-I deficiency versus other patients. This indicated that GH-IGF-I play an important role in the multi-factorial etiology leading to osteopenia/osteoporosis in these patients. GH and IGF-I stimulate the production of OPG and its accumulation in the bone matrix. Alterations in the RANK/RANKL/OPG system in favor of osteoclasts are characteristic in thalassemia due to complicated not clearly delineated mechanisms including chronic anemia, iron toxicity and endocrine complications. Our thalassemic adults had significantly higher osteoclastic bone destruction as evidenced by significantly elevated CCT1 in their serum. The level of serum alkaline phosphatase levels, bone formation marker, did not differ compared to controls, this proposed that the etiology of decreased bone mineral density in these patients is due to increased bone resorption in the presence of attenuated anabolic action due to decreased IGF-I. Other studies in adults and children supported the anabolic effect of IGF-I on bones of thalassemic patients.[9,10,29,49,51,52]
The lack of correlation between the BMD T scores and age may be explained by the more prominent effects of other pathological factors (e.g., IGF-I deficiency and others) compared to the effect of aging in the the etiology of bone disease in these patients. In our patients, chelation therapy with desferrioxamine since early childhood before the introduction of oral iron chelators, may have contributed to their bone disease through inhibiting DNA synthesis, osteoblast and fibroblast proliferation, osteoblast precursor's differentiation, collagen formation and enhancing osteoblast apoptosis.
Limitations of the study
We assessed the GH secretion after a single stimulation test. Pincelli et al.,[6] have shown that GHD in TM patients can be overestimated if assessed by a single GH provocative test, suggesting that two dynamic tests should be performed as for non thalassaemic patients. In addition, the cardiac function in these TM patients with GHD was not fully evaluated.
In conclusion, this study confirms the necessity to screen the status of GH/IGF-I axis in adult patients with TM. Our thalassemic patients with GH and/or IGF-I deficiency were significantly shorter and had lower BMD at lumbar spine compared to those with normal GH-IGF-I axis. Clonidine test appears to be effective and safe in adults. Further studies are needed to evaluate the effect of GH therapy bone mineral density and heart function patients with TM and severe GHD.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Toumba M, Sergis A, Kanaris C, Skordis N. Endocrine complications in patients with Thalassaemia Major. Pediatr Endocrinol Rev. 2007;5:642–8. [PubMed] [Google Scholar]
- 2.Delvecchio M, Cavallo L. Growth and endocrine function in thalassemia major in childhood and adolescence. J Endocrinol Invest. 2010;33:61–8. doi: 10.1007/BF03346551. [DOI] [PubMed] [Google Scholar]
- 3.Vidergor G, Goldfarb AW, Glaser B, Dresner-Pollak R. Growth hormone reserve in adult beta thalassemia patients. Endocrine. 2007;31:33–7. doi: 10.1007/s12020-007-0018-7. [DOI] [PubMed] [Google Scholar]
- 4.Scacchi M, Danesi L, Cattaneo A, Valassi E, Pecori Giraldi F, Argento C, et al. Growth hormone deficiency in adult thalassaemic patients. Clin Endocrinol (Oxf) 2007;67:790–5. doi: 10.1111/j.1365-2265.2007.02965.x. [DOI] [PubMed] [Google Scholar]
- 5.La Rosa C, De Sanctis V, Mangiagli A, Mancuso M, Guardabasso V, Galati MC, et al. Growth hormone secretion in adult patients with thalassaemia. Clin Endocrinol (Oxf) 2005;62:667–71. doi: 10.1111/j.1365-2265.2005.02276.x. [DOI] [PubMed] [Google Scholar]
- 6.Pincelli AI, Masera N, Tavecchia L, Perotti M, Perra S, Mariani R, et al. GH deficiency in adult B-thalassemia major patients and its relationship with IGF-1 production. Pediatr Endocrinol Rev. 2011;8(Suppl 2):S284–9. [PubMed] [Google Scholar]
- 7.Poggi M, Pascucci C, Monti S, Pugliese P, Lauri C, Amodeo G, et al. Prevalence of growth hormone deficiency in adult polytransfused β-thalassemia patients and correlation with transfusional and chelation parameters. J Endocrinol Invest. 2010;33:534–8. doi: 10.1007/BF03346643. [DOI] [PubMed] [Google Scholar]
- 8.Rosen T, Wilhelmsen L, Landin-Wilhelmsen K, Lappas G, Lindstedt G, Bengtsson BA. Increased fracture frequency in adult subjects with hypopituitarism and GH deficiency. Eur J Endocrinol. 1997;137:240–5. doi: 10.1530/eje.0.1370240. [DOI] [PubMed] [Google Scholar]
- 9.Scacchi M, Danesi L, Cattaneo A, Valassi E, Pecori Giraldi F, Argento C, et al. Bone demineralization in adult thalassaemic patients: Contribution of GH and IGF-I at different skeletal sites. Clin Endocrinol (Oxf) 2008;69:202–7. doi: 10.1111/j.1365-2265.2008.03191.x. [DOI] [PubMed] [Google Scholar]
- 10.Soliman AT, El Banna N, Abdel Fattah M, ElZalabani MM, Ansari BM. Bone mineral density in prepubertal children with beta-thalassemia: Correlation with growth and hormonal data. Metabolism. 1998;47:541–8. doi: 10.1016/s0026-0495(98)90237-2. [DOI] [PubMed] [Google Scholar]
- 11.Glynn N, Agha A. Diagnosing growth hormone deficiency in adults. Int J Endocrinol 2012. 2012 doi: 10.1155/2012/972617. 972617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraser NC, Seth J, Brown NS. Clonidine is a better test for growth hormone deficiency than insulin hypoglycaemia. Arch Dis Child. 1983;58:355–8. doi: 10.1136/adc.58.5.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pirinççioğlu AG, Akpolat V, Köksal O, Haspolat K, Söker M. Bone mineral density in children with beta-thalassemia major in Diyarbakir. Bone. 2011;49:819–23. doi: 10.1016/j.bone.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Mahachoklertwattana P, Chuansumrit A, Sirisriro R, Choubtum L, Sriphrapradang A, Rajatanavin R. Bone mineral density, biochemical and hormonal profiles in suboptimally treated children and adolescents with beta-thalassaemia disease. Clin Endocrinol (Oxf) 2003;58:273–9. doi: 10.1046/j.1365-2265.2003.01707.x. [DOI] [PubMed] [Google Scholar]
- 15.Brabant G, von zur Mühlen A, Wüster C, Ranke MB, Kratzsch J, Kiess W, et al. The German KIMS Board. Serum insulin-like growth factor I reference values for an automated chemiluminescence immunoassay system: Results from a multicenter study. Horm Res. 2003;60:53–60. doi: 10.1159/000071871. [DOI] [PubMed] [Google Scholar]
- 16.De Boer H, Block GJ, Van Der Veen EA. Clinical aspects of growth hormone deficiency in adults. Endocr Rev. 1995;16:63–86. doi: 10.1210/edrv-16-1-63. [DOI] [PubMed] [Google Scholar]
- 17.Newmann CB, Kleinberg DL. Adult growth hormone deficiency. Endocrinologist. 1998;8:178–86. [Google Scholar]
- 18.Carrol PV, Christ ER. Growth hormone deficiency in adulthood and the effects of growth hormone replacement: A review. J Clin Endocrinol Metab. 1998;83:382–95. doi: 10.1210/jcem.83.2.4594. [DOI] [PubMed] [Google Scholar]
- 19.De Sanctis V, Skordis N, Galati MC, Raiola G, Giovannini M, Candini G, et al. Growth hormone and adrenal response to intramuscular glucagon test and its relationship to IGF-1 production and left ventricular ejection fraction in adult B-thalassemia major patients. Pediatr Endocrinol Rev. 2011;8(Suppl 2):S290–4. [PubMed] [Google Scholar]
- 20.Soliman AT, Abushahin A, Abohezeima K, Khalafallah H, Adel A, Elawwa A, et al. Age related igf-I changes and igf-I generation in thalassemia major. Pediatr Endocrinol Rev. 2011;8(Suppl 2):S278–83. [PubMed] [Google Scholar]
- 21.Pincelli AI, Masera N, Tavecchia L, Perotti M, Perra S, Mariani R, et al. GH deficiency in adult B-thalassemia major patients and its relationship with IGF-1 production. Pediatr Endocrinol Rev. 2001;(Suppl 2):S284–9. [PubMed] [Google Scholar]
- 22.Soliman AT, El Banna N, Ansari BM. GH response to provocation and circulating IGF-I and IGF-binding protein-3 concentrations, the IGF-I generation test and clinical response to GH therapy in children with beta-thalassaemia. Eur J Endocrinol. 1998;138:394–400. doi: 10.1530/eje.0.1380394. [DOI] [PubMed] [Google Scholar]
- 23.Shehadeh N, Hazani A, Rudolf MC, Peleg I, Benderly A, Hochberg Z. Neurosecretory dysfunction of growth hormone secretion in thalassemia major. Acta Paediatr Scand. 1990;79:790–5. doi: 10.1111/j.1651-2227.1990.tb11556.x. [DOI] [PubMed] [Google Scholar]
- 24.Park EJ, Kim H, Kim Y, Yi J, Choi K, Park K. Inflammatory responses may be induced by a single intratracheal instillation of iron nanoparticles in mice. Toxicology. 2010;275:65–71. doi: 10.1016/j.tox.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Morabito N, Russo GT, Gaudio A, Lasco A, Catalano A, Morini E, et al. The “lively” cytokines network in beta-thalassemia major-related osteoporosis. Bone. 2007;40:1588–94. doi: 10.1016/j.bone.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis. Endocr Rev. 2001;22:53–74. doi: 10.1210/edrv.22.1.0419. [DOI] [PubMed] [Google Scholar]
- 27.Mazza P, Giua R, De Marco S, Bonetti MG, Amurri B, Masi C, et al. Iron overload in thalassemia: Comparative analysis of magnetic resonance imaging, serum ferritin and iron content of the liver. Haematologica. 1995;80:398–404. [PubMed] [Google Scholar]
- 28.Papakonstantinou OG, Maris TG, Kostaridou V, Gouliamos AD, Koutoulas GK, Kalovidouris AE, et al. Assessment of liver iron overload by T2-quantitative magnetic resonance imaging: Correlation of T2-QMRI measurements with serum ferritin concentration and histologic grading of siderosis. Magn Reson Imaging. 1995;13:967–77. doi: 10.1016/0730-725x(95)00041-e. [DOI] [PubMed] [Google Scholar]
- 29.Morabito N, Russo GT, Gaudio A, Lasco A, Catalano A, Morini E, et al. The “lively” cytokines network in β-Thalassemia Major-related osteoporosis. Bone. 2007;40:1588–94. doi: 10.1016/j.bone.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 30.Herrington J, Carter-Su C. Signaling pathways activated by the growth hormone receptor. Trends Endocrinol Metab. 2001;12:252–7. doi: 10.1016/s1043-2760(01)00423-4. [DOI] [PubMed] [Google Scholar]
- 31.May Y, Ling PR, Fitzgibbons TP, McCowen KC, Frick GP, Bistrian BR. Endotoxin-induced inhibition of growth hormone receptor signaling in rat liver in vivo. Endocrinology. 1999;140:5505–15. doi: 10.1210/endo.140.12.7212. [DOI] [PubMed] [Google Scholar]
- 32.De Benedetti F, Alonzi T, Moretta A, Lazzaro D, Costa P, Poli V, et al. Interleukin 6 causes growth impairment in transgenic mice through a decrease in insulin-like growth factor-I. A model for stunted growth in children with chronic inflammation. J Clin Invest. 1997;99:643–50. doi: 10.1172/JCI119207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Benedetti F, Pignatti P, Vivarelli M, Meazza C, Ciliberto G, Savino R, et al. In vivo neutralization of human IL-6 (hIL-6) achieved by immunization of hIL-6-transgenic mice with a hIL-6 receptor antagonist. J Immunol. 2001;166:4334–40. doi: 10.4049/jimmunol.166.7.4334. [DOI] [PubMed] [Google Scholar]
- 34.De Benedetti F, Meazza C, Oliveri M, Pignatti P, Vivarelli M, Alonzi T, et al. Effect of IL-6 on IGF binding protein-3: A study in IL-6 transgenic mice and in patients with systemic juvenile idiopathic arthritis. Endocrinology. 2001;142:4818–26. doi: 10.1210/endo.142.11.8511. [DOI] [PubMed] [Google Scholar]
- 35.Baldini M, Forti S, Marcon A, Ulivieri FM, Orsatti A, Tampieri B, et al. Endocrine and bone disease in appropriately treated adult patients with beta-thalassemia major. Ann Hematol. 2010;89:1207–13. doi: 10.1007/s00277-010-1007-0. [DOI] [PubMed] [Google Scholar]
- 36.Jensen CE, Tuck SM, Agnew JE, Koneru S, Morris RW, Yardumian A, et al. High prevalence of low bone mass in thalassaemia major. Br J Haematol. 1998;103:911–5. doi: 10.1046/j.1365-2141.1998.01108.x. [DOI] [PubMed] [Google Scholar]
- 37.Mahachoklertwattana P, Pootrakul P, Chuansumrit A, Choubtum L, Sriphrapradang A, Sirisriro R, et al. Association between bone mineral density and erythropoiesis in Thai children and adolescents with thalassemia syndromes. J Bone Miner Metab. 2006;24:146–52. doi: 10.1007/s00774-005-0661-0. [DOI] [PubMed] [Google Scholar]
- 38.De Boer H, Block GJ, Van Der Veen EA. Clinical aspects of growth hormone deficiency in adults. Endocr Rev. 1995;16:63–86. doi: 10.1210/edrv-16-1-63. [DOI] [PubMed] [Google Scholar]
- 39.Wuster C, Slenczka E, Ziegler R. Increased prevalence of osteoporosis and arteriosclerosis in conventionally substituted anterior pituitary insufficiency: Need for additional growth hormone substitution? Klin Wochenschr. 1991;69:769–73. doi: 10.1007/BF01797616. [DOI] [PubMed] [Google Scholar]
- 40.Rosen T, Wilhelmensem L, Landin-Wilhelsem K, Lappas G, Bengtsson BA. Increased fracture frequency in adult patients with hypopituitarism and GH deficiency. Eur J Endocrinol. 1997;137:240–5. doi: 10.1530/eje.0.1370240. [DOI] [PubMed] [Google Scholar]
- 41.De Boer H, Blok GJ, Van Lingen A, Teule GJ, Lips P, van der Veen EA. The consequences of childhood-onset growth hormone deficiency for adult bone mass. J Bone Miner Res. 1994;9:1319–26. doi: 10.1002/jbmr.5650090822. [DOI] [PubMed] [Google Scholar]
- 42.Kaufmann JM, Tachman P, Vermeulen A, Vandeweghe M. Bone mineral status in growth hormone deficient males with isolated and multiple pituitary deficiencies of childhood onset. J Clin Endocrinol Metab. 1992;74:118–23. doi: 10.1210/jcem.74.1.1727808. [DOI] [PubMed] [Google Scholar]
- 43.Holmes SJ, Economou G, Whitehouse RW, Adams JE, Shalet SS. Reduced bone mineral density in patients with adult onset growth hormone deficiency. J Clin Endocrinol Metab. 1994;78:669–74. doi: 10.1210/jcem.78.3.8126140. [DOI] [PubMed] [Google Scholar]
- 44.Wuster C. Growth hormone and bone metabolism. Acta Endocrinol (Copenh) 1993;128(Suppl 2):S14–8. [PubMed] [Google Scholar]
- 45.Inzucchi SE, Robbins RJ. Effects of growth hormone on human biology. J Clin Endocrinol Metab. 1994;79:691–4. doi: 10.1210/jcem.79.3.8077348. [DOI] [PubMed] [Google Scholar]
- 46.Newmann CB, Kleinberg DL. Adult growth hormone deficiency. Endocrinologist. 1998;8:178–86. [Google Scholar]
- 47.Carrol PV, Christ ER. Growth hormone deficiency in adulthood and the effects of growth hormone replacement: A review. J Clin Endocrinol Metab. 1998;83:382–95. doi: 10.1210/jcem.83.2.4594. [DOI] [PubMed] [Google Scholar]
- 48.Bacharach LK. Bone mineral, histomorphometry, and body composition in adults with growth hormone receptor deficiency. J Bone Miner Res. 1998;13:415–21. doi: 10.1359/jbmr.1998.13.3.415. [DOI] [PubMed] [Google Scholar]
- 49.Amato A, Carella C, Fazio F, La Montagna G, Cittadini A, Sabatini D, et al. Body composition, bone metabolism and heart structure and function in growth hormone deficient adult before and after GH replacement therapy at low doses. J Clin Endocrinol Metab. 1993;77:1671–6. doi: 10.1210/jcem.77.6.8263158. [DOI] [PubMed] [Google Scholar]
- 50.Dresner Pollack R, Rachmilewitz E, Blumenfeld A, Idelson M, Goldfarb AW. Bone mineral metabolism in adults with beta-thalassaemia major and intermedia. Br J Haematol. 2000;111:902–7. [PubMed] [Google Scholar]
- 51.Tantawy AA, El Kholy M, Moustafa T, Elsedfy HH. Bone mineral density and calcium metabolism in adolescents with beta-thalassemia major. Pediatr Endocrinol Rev. 2008;6(Suppl 1):S132–5. [PubMed] [Google Scholar]
- 52.De Sanctis V, Pinamonti A, Di Palma A. Growth and development in thalassaemia major patients with severe bone lesions due to desferrioxamine. Eur J Pediatr. 1996;155:368–72. doi: 10.1007/BF01955263. [DOI] [PubMed] [Google Scholar]


