Abstract
Endoscopy and endoscopic ultrasound (EUS) play a critical role in the detection and management of subepithelial lesions of the gastrointestinal tract. The most common subepithelial lesions detected by endoscopists are gastrointestinal stromal tumors (GISTs), leiomyomas, lipomas, granular cell tumors (GCTs), pancreatic rests and carcinoid tumors. These lesions can be classified based on unique histochemical staining and the gastrointestinal layer of origin. While the majority of the lesions are considered benign, some tumors such as GISTs and carcinoids have a strong propensity for malignant transformation. Therefore, appropriate endoscopic versus surgical management based on size and location is crucial in the prevention of malignant transformation and metastasis. In this review, we provide a systematic approach to the diagnosis, management and treatment of commonly encountered subepithelial lesions.
Keywords: subepithelial lesions, submucosal tumors, subepithelial neoplasms, endoscopic ultrasound (EUS), gastrointestinal stromal tumors (GISTs), carcinoid tumors
Introduction
Subepithelial lesions of the gastrointestinal (GI) tract are commonly encountered during routine esophagogastroduodenoscopy (EGD) and colonoscopy. At least 1% of all EGD examinations diagnose a subepithelial lesion [Hedenbro et al. 1991]. They most commonly occur within the stomach, but are also regularly noted in the esophagus and duodenum. In addition, subepithelial lesions during colonoscopy are often detected. They mostly occur in the rectum and cecum, but familiar lesions such as lipomas may be seen in any part of the colon. This review provides an overview of common subepithelial lesions and aims to provide a systematic approach to diagnosis, management and surveillance.
The overwhelming majority of subepithelial lesions are benign at the time of diagnosis, with fewer than 15% of lesions found to be malignant at presentation [Polkowksi, 2005]. Despite this significant imbalance between worrisome and nonworrisome lesions, many of these neoplasms have potential for malignant transformation and spread to distant organs. The key in evaluating patients with subepithelial lesions is to make a concerted effort at definitively diagnosing the type of lesion possessed by patients so that they can be guided in terms of possible therapies, prognosis, need for surveillance, etc.
Subepithelial lesions are often incidentally discovered and frequently do not explain the indication for which the patient is undergoing the endoscopic examination. If symptoms do exist, chronic anemia from intermittent GI bleeding is most common [Humphris and Jones, 2008]. Other symptoms include abdominal pain and luminal obstruction, depending on the site and size of the lesion. Men and women are equally affected, and most patients are more than 50 years old at the time of diagnosis. Since most of these lesions are smaller than 2 cm in size, computerized tomography (CT) and magnetic resonance imaging (MRI) are often not sensitive enough to discover these lesions within the wall of the GI tract. Endoscopic ultrasound (EUS) is the gold standard for evaluation of subepithelial lesions, as it affords the following benefits: ability to differentiate extramural compression from intramural growth, determine layer of origin, accurately size the lesion, evaluate for regional lymphadenopathy, obtain tissue for diagnosis and help determine appropriate management.
Table 1 lists the 10 commonest types of subepithelial lesions. Most frequently encountered are the gastrointestinal stromal tumor (GIST), leiomyoma, lipoma, pancreatic rest (heterotopic pancreas tissue), carcinoid tumor and inflammatory fibroid lesion. GIST lesions and leiomyomas are mesenchymal tumors arising from muscular spindle-shaped cells. They most commonly originate from the fourth layer of the GI tract wall (muscularis propria), but may be seen arising from the more superficial muscularis mucosa layer as well. Lipomas, fibroids and pancreatic rest lesions tend to be restricted to the submucosal layer of the GI tract, as opposed to duplication cysts which may be seen involving any layer from mucosa to serosa. Carcinoids and granular cell tumors mainly involve the submucosa, but they often grow upward towards the mucosa. This is why they are often diagnosed on routine mucosal biopsy sampling at the time of an index EGD or colonoscopy. Less common lesions such as the lymphangioma and other neural-based tumors (e.g. Schwannoma) mostly exist within the submucosa.
Table 1.
Common subepithelial lesions and degree of mucosal involvement.
| Pathology | Muscularis mucosa | Submucosa | Muscularis propria | Serosa |
|---|---|---|---|---|
| GIST | x | X | ||
| Leiomyoma | x | X | ||
| Lipoma | X | |||
| Granular cell tumor | X | X | ||
| Pancreatic rest | X | |||
| Carcinoid (NET) | X | |||
| Duplication cyst | X | X | X | X |
| Fibroid lesion | X | |||
| Varices | X | |||
| Lymphangioma | X | |||
| Neural tumors (e.g. Schwannoma) | X | X |
GIST, gastrointestinal stromal tumor; NET, neuroendocrine tumor.
GIST
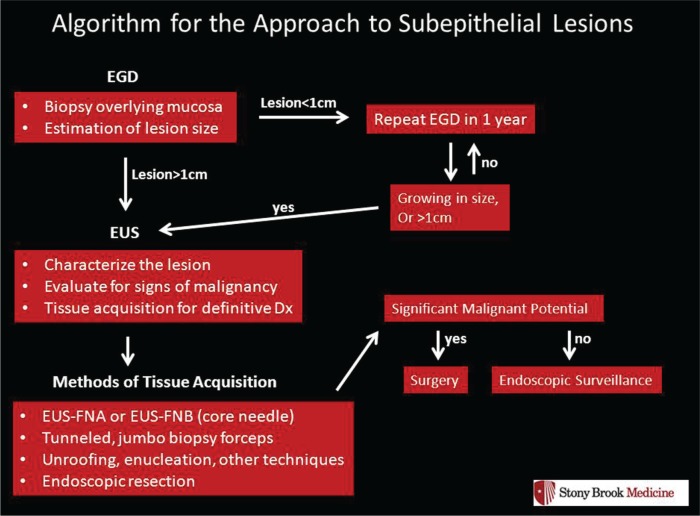
GIST lesions originate from the interstitial cells of Cajal. In terms of the pathophysiology of this neoplasm, there is a gain of function mutation in the KIT gene that codes for the c-kit protein, a tyrosine kinase receptor [Hirota et al. 1998]. Immunohistochemical (IHC) staining of these tumors is CD117 positive in nearly 95% of cases, which corresponds to the activation of c-kit as seen in Figure 1a. All GIST lesions have the potential for malignant transformation and distant metastases.
Figure 1.
(a) Immunohistochemical staining positive for CD117 (KIT). (b) EUS view of a hypoechoic subepithelial lesion arising from the fourth layer, muscularis propria consistent with a GIST.
EUS, endoscopic ultrasound; GIST, gastrointestinal stromal tumor.
The risk of malignancy with GIST lesions is dependent on the size of the lesion and the number of cells noted to be undergoing mitosis at pathological evaluation. For example, lesions larger than 5 cm in size, and with a mitotic count of >10/50 per high power field (HPF) have the highest likelihood of malignancy at the time of diagnosis. Table 2 summarizes the association between risk of malignancy, size and mitotic count. Location is important as well. GIST lesions in the small bowel have a much higher risk of malignancy than those seen, for example, within the stomach or rectum [Grotz and Donohue, 2011].
Table 2.
Risk of malignancy in GIST lesions.
| Risk of malignancy | Size | Mitotic count |
|---|---|---|
| Very low | <2 cm | <5/50 HPF |
| Low | 2–5 cm | <5/50 HPF |
| Moderate | >5 cm | 6–10/50 HPF |
| >5 cm | <5/50 HPF | |
| High | >5 cm | 6–10/50 HPF |
| Any size | >10/50 HPF |
HPF, high power field.
The management of GIST lesions in the upper and lower GI tract discovered during EGD and colonoscopy is dependent on size and the presence of symptoms. Suspected GIST lesions of at least 1 cm in size should be referred for EUS evaluation. Frequently the results of EUS with fine needle aspiration (FNA), however, do not yield enough cellular material for the cytopathologist to make an accurate assessment of mitotic count. This is mainly achieved by the surgical pathologist once a tumor has been removed. As a result, the management is most often guided by the size of the lesion and whether or not the patient is felt to be symptomatic due to the lesion itself (e.g. history of iron-deficiency anemia and the presence of an ulcerated GIST with stigmata of recent bleeding). For tumors that are causing symptoms, patients should undergo surgical resection so that these lesions may be totally removed. In addition, tumors noted within the small bowel (jejunum or ileum) should be referred for surgical resection as well because of the higher risk of malignancy and the high likelihood of symptoms associated with these tumors (which is commonly the investigational indication that discovered the tumor in the first place). Large tumors >2 cm in size should also be referred for resection—even if the patient is asymptomatic. Also, patients with high-risk lesions should be referred to a medical oncologist for consideration of adjuvant therapy with one of the tyrosine kinase inhibitor drugs (e.g. Imatinib or Gleevec™).
As per National Comprehensive Cancer Network (NCCN) guidelines, smaller GIST lesions (<2 cm in size) in patients who are asymptomatic and without any high-risk features on endoscopic examination lesions may be closely followed with annual EGD and/or EUS exam. Surgery may be pursued if the lesion is noted to be growing during the surveillance period. In addition, it may be reasonable to consider surgical resection in this group of patients with smaller lesions if the patient wishes for it to be removed and their operative risks are relatively low. Lastly, most GIST lesions should not undergo attempted endoscopic resection. As most of these lesions arise from the muscularis propria layer of the GI tract wall, (the last layer before the serosa), complete endoscopic removal is not achievable without perforation or full-thickness resection. Figure 1b provides an endoscopic ultrasound view of a GIST, seen as a hypoechoic lesion arising from the muscularis propria.
Leiomyoma
Leiomyomas also originate from the muscular layers of the GI tract wall. Most commonly they arise from the muscularis propria, but some are seen within the muscularis mucosa. Their location is slightly different from GIST lesions, and most commonly they are seen in the mid or distal esophagus. IHC staining is negative for CD117, CD34 and s100, but positive for desmin and α-smooth muscle actin protein. The risk of transformation to a malignant leiomyosarcoma is quite rare [Humphris and Jones, 2008].
Suspected leiomyomas >1 cm in size should be referred for EUS in an effort to confirm the diagnosis. For small lesions of <1–2 cm in size, surveillance EGD and/or EUS may be performed annually if the patient is asymptomatic [Lee et al. 2004]. Conversely, surgical resection is advised for lesions that are causing symptoms (e.g. bleeding), or lesions that are enlarging or displaying structural changes during surveillance exams. Lastly, for small lesions <2 cm in size that arise from either the muscularis mucosa or propria layer on EUS examination, complete endoscopic resection may be performed. Complete resection of these small lesions is associated with a high success rate and almost no complications such as bleeding or perforation [Chun et al. 2013].
Lipoma
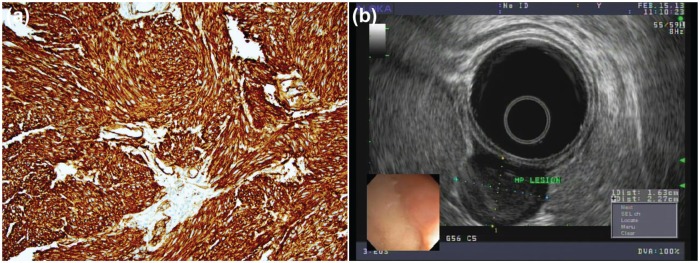
Lipomas are common subepithelial tumors that originate from within the submucosal layer of the GI tract wall. They are most often noted in the gastric antrum and colon, but they also occur frequently in the small bowel. They tend to have a yellow hue on initial endoscopic inspection, and when prodded with a biopsy forceps, they are soft and deformable with a classic ‘pillow’ sign. In fact, demonstration of a positive pillow sign on a subepithelial lesion of yellowish discoloration within the gastric antrum or colon is approximately 98% specific for a lipoma [Hwang et al. 2005]. However, a pancreatic rest may also display a pillow sign, and thus these authors do not believe this finding precludes taking biopsies of suspected lipomas. Figure 2a provides an endoscopic view of a lipoma with overlying mucosa, while Figure 2b shows the classic pillow sign.
Figure 2.
(a) The classic pillow sign, considered 98% specific for lipomas. (b) Fatty submucosal tumor protruding after the overlying mucosa has been removed.
Lipomas have essentially no malignant potential. Therefore once definitively diagnosed, they do not require regular endoscopic surveillance. Since these lesions arise from the submucosa, bite-on-bite biopsies will often reveal grossly yellow adipose tissue once the overlying mucosal layer is unroofed. Utilization of jumbo biopsy forceps for suspected lipomas has been shown to be effective for easy definitive diagnosis, as the underlying submucosal fatty tissue is readily revealed as seen in Figure 2b [Buscaglia et al. 2012]. Jumbo forceps can fit down the channel of standard upper endoscope and do not require the use of a therapeutic endoscope. Endoscopists utilizing this technique need to be skilled at deploying endoscopic clips, as there may be some bleeding once the mucosal surface is removed.
Granular cell tumor
Granular cell tumors (GCTs) are subepithelial lesions of Schwann cell origin. They mostly arise from within the submucosal layer of the GI tract wall and grow towards the mucosa, often making it feasible to obtain a definitive diagnose by means of standard biopsy with a regular forceps. Most GCTs occur within the esophagus. The risk of malignancy is extremely low. In one study, the rate of malignancy was approximately 2–4% at the time of diagnosis and all of these malignant lesions were >4 cm in size [Orlowska et al. 1993].
Small GCTs <1 cm in size may undergo annual EGD surveillance. For larger lesions >2 cm, surgical resection is advised. Intermediate-sized GCTs of 1–2 cm may undergo annual surveillance, or possibly endoscopic resection provided the lesion does not penetrate deeper than the submucosal layer on EUS evaluation.
Pancreatic rest
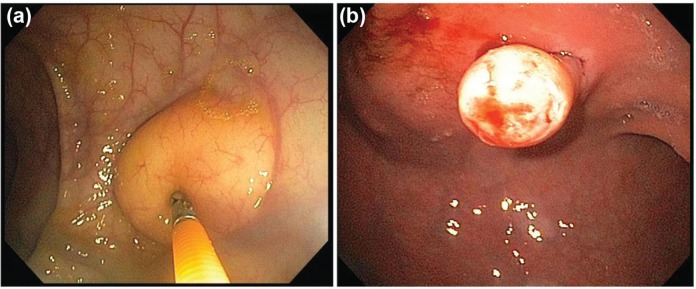
Heterotopic pancreas tissue (pancreatic rest) is quite common and noted in roughly 1–2% of patients in autopsy series. Nearly all of these lesions (90%) are located within the stomach and most often in the gastric antrum. Figure 3a demonstrates ectopic pancreatic tissue arising from within the submucosal layer of the stomach wall. Patients can develop clinical symptoms due to a pancreatic rest, including ulceration and pain, bleeding, acute pancreatitis and gastric outlet obstruction. Endoscopically, they frequently display a central area of umbilication within the center of the lesion as seen in Figure 3b. When submucosal tissue is obtained on deep biopsy sampling, there is obvious pancreatic acinar tissue present histologically. These lesions are considered benign though there have been rare cases of malignant transformation of gastric heterotropic pancreas tissue [Sadeghi et al. 2008]. As these instances are exceedingly rare, these lesions need not require endoscopic surveillance or removal once definitively diagnosed.
Figure 3.
(a) Ectopic pancreatic acinar cells located submucosally in the gastric antrum. (b) Pancreatic tissue with central umbilication, a defining characteristic of pancreatic rest.
Carcinoid tumor
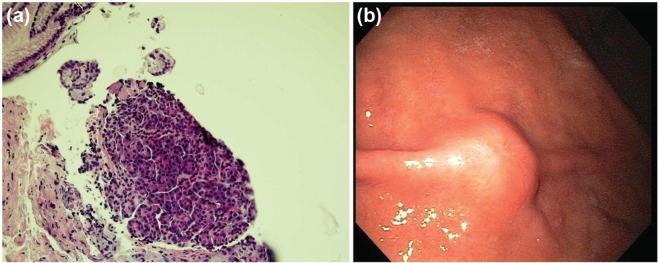
Carcinoid tumors are the most common neoplasm of the small intestine and at least 25% of all carcinoid tumors occur within the small bowel. Their location is most frequent within the ileum, followed by the jejunum and the duodenum. There tends to be a slight female predominance with a male:female ratio of 1:1.6. These lesions usually originate from the mucosal layer of the GI tract wall and penetrate into the submucosal layer. As a result, they are often diagnosed on routine mucosal biopsy sampling at the time of an index EGD or colonoscopy. Figures 4a and 4b demonstrate an endoscopic view of a smooth, polypoid carcinoid tumor of the duodenum, originating in the mucosal layer of the GI tract.
Figure 4.
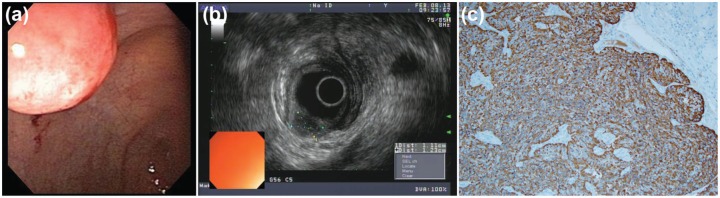
(a) Smooth, polypoid-like carcinoid tumor in the duodenum. (b) EUS view of a hypoechoic carcinoid tumor originating from the mucosal layer of the gastrointestinal tract with penetration through the submucosal layer. (c) Well-differentiated neuroendocrine neoplasm, synaptophysin stain positive.
EUS, endoscopic ultrasound.
Gastric carcinoid tumors are commonly diagnosed on routine EGD and account of 9% of all carcinoid tumors [Modlin et al. 2004]. There are three subtypes of gastric carcinoids with varying levels of malignant potential:
Type I gastric carcinoid tumors are associated with atrophic gastritis, pernicious anemia and hypergastrinemia. These tumors have a very low potential for malignant transformation.
Type II gastric carcinoid tumors are also associated with hypergastrinemia, but the high gastrin levels are due to Zollinger–Ellison Syndrome or MEN-1 (multiple endocrine neoplasia syndrome, type 1). These tumors have an intermediate potential for malignant transformation.
Type III gastric carcinoid tumors are the sporadic form and are not associated with high gastrin levels. These tumors have a high potential for malignant transformation.
It is critical to make a concerted effort at diagnosing the exact type of gastric carcinoid so that patients are managed appropriately and provided with the most accurate idea of prognosis. For Type I and Type II lesions (hypergastrinemia), endoscopic resection is appropriate when the tumor is <1–2 cm in size. Snare cautery polypectomy technique should be utilized, often with preceding submucosal injection of fluid to be sure to lift the entire lesion off the underlying muscle layer and assure complete removal of the base of the tumor. For larger tumors >2 cm or for multiple tumors, surgical resection is advised. This is often accompanied by distal antrectomy to remove the G cell mass burden, or fundectomy to remove the enterochromaffin-like (ECL) cell mass burden. Following resection of these tumors, surveillance EGD should be performed every 6–12 months for the first 3 years and annually thereafter [Kulke et al. 2012].
Type III gastric carcinoid tumors (normal gastrin levels) are the sporadic form and should not be removed endoscopically. Surgical resection is recommended with lymph node dissection; preferably by an experienced upper GI surgical oncologist [Ramage et al. 2005]. Again, surveillance EGD exam should be performed following the operation.
Rectal carcinoid tumors are frequently discovered during routine screening colonoscopy. Following initial EUS examination, small lesions <1 cm in size that are confined to the submucosa should be removed endoscopically. Lesions >2 cm, or with penetration into the muscularis propria layer on EUS, or associated with enlarged regional lymphadenopathy should be referred for surgical resection by an experienced colorectal surgeon [Kobayashi et al. 2005]. MRI of the pelvis is also recommended for these patients in planning disease management. For intermediate-sized lesions (1–2 cm), endoscopic resection may be performed if the lesion is confined to the submucosa and there are no other concerning features on EUS and/or MRI. Alternatively, these lesions may also be referred for surgical resection, including some minimally invasive transanal resection techniques. Once resected, follow-up endoscopies are recommended at 6 and 12 months after therapy as per NCCN guidelines [Kulke et al. 2012].
There are no specific guidelines or recommendations in place for the management of duodenal carcinoid tumors. For nonampullary duodenal carcinoid tumors, it is reasonable to manage them in similar fashion as the rectal carcinoid tumors. For ampullary carcinoid tumors, small lesions (<1–2 cm) may be removed with endoscopic ampullectomy by an experienced pancreaticobiliary endoscopist, as long as an initial EUS examination shows no suggestion of advanced disease (regional lymphadenopathy, growth into the muscularis propria layer, encroachment of tumor up the distal common bile duct, etc.). Larger or more advanced tumors should be referred for surgical resection, including surgical ampullectomy or pancreaticoduodenectomy (Whipple procedure).
In addition to endoscopic surveillance post resection, patients with carcinoid tumors should have a complete history and physical examination to evaluate for recurrence. These patients should be reevaluated 3–12 months after resection, then annually if there are no signs of recurrence. Additionally, chromogranin A, a serum tumor marker, may be monitored for tumor recurrence while 5-hydroxyidoleacetic acid (5-HIAA), a metabolite of serotonin, may be monitored for treatment response [Kulke et al. 2012].
Algorithmic approach
Figure 5 outlines an algorithm that can be used for the approach to subepithelial lesions of the upper and lower GI tract. At the time of initial EGD or colonoscopy, biopsy of the overlying mucosa of the tumor should be performed in all cases. Frequently a definitive diagnosis can be made with simple mucosal biopsies. Not too infrequently, an unexpected diagnosis such as adenocarcinoma may be encountered as well. The biopsy forceps should also be used to estimate the size of the lesion. For small lesions (<1 cm in size), a repeat EGD exam may be performed in 1 year to re-evaluate the tumor and assure no significant growth or morphological changes. EUS of these small lesions is not mandatory.
Figure 5.
Algorithm for the approach to subepithelial lesions (adapted from Humphris and Jones, 2008).
EGD, esophagogastroduodenoscopy; EUS, endoscopic ultrasound; FNA, fine needle aspiration; FNB, fine needle biopsy.
For tumors that measure >1 cm in size, an initial EUS examination should be performed to better characterize the lesion, to evaluate for signs of malignancy, and to perform various methods of tissue acquisition in effort to make a definitive diagnosis. Methods of more complex tissue acquisition may include EUS-FNA or EUS-FNB (fine needle biopsy with a core needle), tunneled biopsies with a jumbo forceps, partial endoscopic resection or enucleation.
Once a diagnosis is made, the risk of possible malignant transformation should be addressed. Further management is dependent upon this risk. For example, a large carcinoid tumor should be removed (surgically or endoscopically depending on the size, type and location of tumor as outline above). Small tumors with malignant potential may undergo endoscopic surveillance (e.g. 1.5 cm GIST lesion or granular cell tumor). Benign lesions without any significant risk of malignancy may be disregarded (e.g. lipoma, pancreatic rest). Lastly, any significant growth or morphological change in a lesion should prompt further investigation with EUS and/or cross-sectional imaging if necessary.
In regards to an endoscopic versus surgical approach to the removal of subepithelial lesions, the decision is guided primarily by size, location and layer of origin within the GI tract wall. For example, lesions involving the submucosa (e.g. small carcinoids and GCTs) are more likely to undergo complete resection by means of endoscopic mucosal resection (EMR) and/or endoscopic submucosal dissection (ESD) with minimal complication. However, lesions involving the muscularis propria layer are generally referred for surgical resection, as the ability to achieve complete endoscopic resection may be difficult without causing perforation through the serosal layer of the GI tract wall. Thus, the role of EUS is invaluable in these lesions for determining the degree of wall layer involvement, and ultimately the appropriate management.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflict of interest in preparing this article.
Contributor Information
Laila Menon, Department of Medicine, Division of Gastroenterology, School of Medicine, State University of New York (SUNY) at Stony Brook, Stony Brook, New York, USA.
Jonathan M. Buscaglia, Division of Gastroenterology, Stony Brook University Hospital, 100 Nicolls Road, HSC-Level 17, Room 060, Stony Brook, New York 11790, USA
References
- Buscaglia J., Nagula S., Jayaraman V., Robbins D., Vadada D., Gross S., et al. (2012) Diagnostic yield and safety of jumbo biopsy forceps in patients with subepithelial lesions of the upper and lower GI tract. Gastrointest Endosc 75: 1147–1152 [DOI] [PubMed] [Google Scholar]
- Chun S., Kim K., Park D., Lee I., Park J., Moon S., et al. (2013) Endoscopic submucosal dissection as a treatment for gastric subepithelial tumors that originate from the muscularis propria layer: a preliminary analysis of appropriate indications. Surgical Endosc 27: 3271–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetri G., Benjamin R., Blanke C., Blay J., Casali P., Choi H., et al. (2007). NCCN Task Force Report: management of patients with gastrointestinal stromal tumor (GIST)-update of the NCCN. J Natl Compr Canc Netw 2007; 5(Suppl 2): S1–29 [PubMed] [Google Scholar]
- Grotz T., Donohue J. (2011) Surveillance strategies for gastrointestinal stromal tumors.J Surgical Oncol 104: 921–927 [DOI] [PubMed] [Google Scholar]
- Hedenbro J., Ekelund M., Wetterberg P. (1991) Endoscopic diagnosis of submucosal gastric lesions. J Surg Endosc 1: 20–23 [DOI] [PubMed] [Google Scholar]
- Hirota S., Isozaki K., Moriyama Y., Hashimoto K., Nishida T., Ishiguro S., et al. (1998) Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279: 577–580 [DOI] [PubMed] [Google Scholar]
- Humphris J., Jones D. (2008) Subepithelial mass lesions in the upper gastrointestinal tract. J Gastroenterol Hepatol 23: 556–566 [DOI] [PubMed] [Google Scholar]
- Hwang J., Saunders M., Rulyak S., Shaw S., Nietsch H., Kimmey M., et al. (2005) A prospective study comparing endoscopy and EUS in the evaluation of GI subepithelial masses. Gastrointest Endosc 62: 202–208 [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Katsumata T., Yoshizawa S., Sada M., Igarashi M., Saigenji K., et al. (2005) Indications for endoscopic polypectomy for rectal carcinoid tumors and clinical usefulness of endoscopic ultrasound. Dis Colon Rectum 48: 285–291 [DOI] [PubMed] [Google Scholar]
- Kulke M., Benson A., Bergsland E., et al. (2012) NCCN Task Force Report. Neuroendocrine tumors. J Natl Compr Canc Netw 10: 724–764 [DOI] [PubMed] [Google Scholar]
- Lee L., Singhal S., Brinster C., Marshall B., Kochman M., Kaiser L., et al. (2004) Current management of esophageal leiomyoma. J Am Coll Surg 198: 136–146 [DOI] [PubMed] [Google Scholar]
- Modlin I., Lye K., Kidd M. (2004) A 50-year analysis of 562 gastric carcinoids: small tumor or larger problem? Am J Gastroenterol 99: 23–32 [DOI] [PubMed] [Google Scholar]
- Orlowska J., Pachiewski J., Gugulski A., Butruk E. (1993) A conservative approach to granular cell tumors of the esophagus: four case reports and literature review. Am J Gastroenterol 88: 311–315 [PubMed] [Google Scholar]
- Polkowski M. (2005) Endoscopic ultrasound and endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy 37: 635–645 [DOI] [PubMed] [Google Scholar]
- Ramage J., Davies A., Ardill J., et al. (2005) Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumors. Gut 54: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi N., Godambe A., Shienbaum A., Alloy A. (2008) Premalignant gastric heterotopic tissue. Gastroenterol Hepatol 4: 218–221 [PMC free article] [PubMed] [Google Scholar]