Abstract
Background and Purpose
Neuronal GABAA receptors are pentameric chloride ion channels, which include synaptic αβγ and extrasynaptic αβδ isoforms, mediating phasic and tonic inhibition respectively. Although the subunit arrangement of αβγ receptors is established as β-α-γ-β-α, that of αβδ receptors is uncertain and possibly variable. We compared receptors formed from free α1, β3 and δ or γ2L subunits and concatenated β3-α1-δ and β3-α1 subunit assemblies (placing δ in the established γ position) by investigating the effects of R-(+)-etomidate (ETO), an allosteric modulator that selectively binds to transmembrane interfacial sites between β3 and α1.
Experimental Approach
GABA-activated receptor-mediated currents in Xenopus oocytes were measured electrophysiologically, and ETO-induced allosteric shifts were quantified using an established model.
Key Results
ETO (3.2 μM) similarly enhanced maximal GABA (1 mM)-evoked currents in oocytes injected with 5 ng total mRNA and varying subunit ratios, for α1β3(1:1), α1β3δ(1:1:1) and α1β3δ(1:1:3), but this potentiation by ETO was significantly greater for β3-α1-δ/β3-α1(1:1) receptors. Reducing the amount of α1β3δ(1:1:3) mRNA mixture injected (0.5 ng) increased the modulatory effect of ETO, matching that seen with β3-α1-δ/β3-α1(1:1, 1 ng). ETO similarly reduced EC50s and enhanced maxima of GABA concentration-response curves for both α1β3δ and β3-α1-δ/β3-α1 receptors. Allosteric shift parameters derived from these data depended on estimates of maximal GABA efficacy, and the calculated ranges overlap with allosteric shift values for α1β3γ2L receptors.
Conclusion and Implications
Reducing total mRNA unexpectedly increased δ subunit incorporation into receptors on oocyte plasma membranes. Our results favour homologous locations for δ and γ2L subunits in α1β3γ2/δ GABAA receptors.
Keywords: allosteric modulation, etomidate, general anaesthetics, GABAA receptors, δ subunits, concatenated subunit assemblies, electrophysiology
Introduction
GABAA receptors are ligand-gated pentameric chloride ion channels formed by five subunits from among 16 homologous GABAA receptor subunit subtypes (α1–α6, β1–β3, γ1–γ3, δ, ε, π and θ) (Olsen and Sieghart, 2008). GABAA receptors in vivo are predominantly composed of αβδ and αβγ isoforms (McKernan and Whiting, 1996). The αβγ receptors are mainly postsynaptic, mediating phasic neuronal inhibition, whereas αβδ receptors are extrasynaptic, mediating tonic inhibition (Mody and Pearce, 2004; Farrant and Nusser, 2005). The subunit stoichiometry of heterologously expressed αβγ receptors is well established as 2α:2β:1γ (Chang et al., 1996; Tretter et al., 1997), with a counterclockwise subunit arrangement of β-α-γ-β-α viewed from the extracellular space (Baumann et al., 2001). Several studies of recombinant αβδ receptors are consistent with subunit stoichiometry and arrangement similar to those of αβγ receptors (Barrera et al., 2008; Botzolakis et al., 2008; Shu et al., 2012). However, variable subunit stoichiometry and arrangements in expressed αβδ receptors have been suggested by quantitative biochemical studies varying the ratio of subunit-encoding DNAs (Wagoner and Czajkowski, 2010) and functional comparison of αβδ receptors formed from free subunits with various concatenated subunit assemblies (Kaur et al., 2009).
GABAA receptors are positively modulated by many general anaesthetics including propofol, etomidate (ETO), pentobarbital and alphaxalone (Hevers and Luddens, 1998; Feng, 2010; Akk and Steinbach, 2011; Forman and Miller, 2011) and by endogenous neurosteroids such as tetrahydrodeoxycorticosterone (THDOC) (Wohlfarth et al., 2002; Stell et al., 2003; Hosie et al., 2006). Many studies have reported that THDOC increases maximal GABA-activated αβδ receptor-mediated currents (Wallner et al., 2003; Zheleznova et al., 2008; Kaur et al., 2009; Meera et al., 2009; Baker et al., 2010; Baur et al., 2010), with the degree of positive modulation varying from several fold to more than 20-fold. This wide range of effects could reflect variable δ subunit stoichiometry and subunit arrangements producing different numbers and types of THDOC sites (Shu et al., 2012). However, inferences regarding αβδ receptor stoichiometry and arrangement cannot be drawn from THDOC modulation because the structures forming neurosteroid sites on GABAA receptors remain undefined (Akk et al., 2004; Hosie et al., 2006; Bracamontes et al., 2011). In contrast, R-(+)-ETO is known to modulate α1β2/3γ2 GABAA receptors selectively via two binding sites located at transmembrane subunit interfaces between α-M1 and β-M3 domains (Li et al., 2006; Chiara et al., 2012). A quantitative model describing ETO modulation of GABAA receptor activity has been validated for α1β2γ2L receptors formed from free or concatenated subunits and activated with either full or partial agonists (Rusch et al., 2004; Guitchounts et al., 2012). ETO also enhances α4β3δ receptor currents (Brown et al., 2002; Meera et al., 2009), although the number and location of its sites in αβδ receptors remain unexplored.
We hypothesized that if the subunit stoichiometries or arrangements of α1β3γ2 and α1β3δ differ, then the number of α1-M1/β3-M3 interfacial ETO sites on both receptor isoforms would differ, resulting in divergent allosteric effects with bound ETO. We heterologously expressed, in Xenopus oocytes, α1β3δ and α1β3γ2L receptors formed from free subunits as well as concatenated β3-α1-δ/β3-α1 receptors (designed to form pentamers with δ in the same position as γ2L in α1β3γ2L receptors) and compared ETO modulation of these receptors using two microelectrode electrophysiology and quantitative allosteric model analysis.
Our results show that in oocyte-expressed α1β3δ receptors formed from free subunits, modulation of maximal GABA-elicited responses by both ETO and THDOC depends on subunit mRNA ratio and the total mRNA injected. Under expression conditions that optimize allosteric gating modulation, ETO produces similar upward (increased maximum) and leftward (reduced EC50) shifts of GABA concentration-response curves for both free subunit α1β3δ and concatenated β3-α1-δ/β3-α1 receptors. Quantitative allosteric shifts calculated for ETO modulation of both α1β3δ and α1β3γ2L receptors did not differ significantly. Our results indicate that conditions leading to efficient δ subunit incorporation into Xenopus oocyte-expressed GABAA receptors differ from those for γ2L incorporation. They also favour the hypothesis that α1β3δ and α1β3γ2L receptors have a similar number and configuration of ETO sites formed by β3 and α1 subunits.
Methods
Animals and oocyte harvest
Animal procedures were approved by the Institutional Animal Care and Use Committee of Massachusetts General Hospital. Frogs were purchased from Xenopus 1, Dexter, Michigan, and ∼20 frogs were used in these experiments. They were kept in fresh water at 18°C (1-2 frogs per cage). The room was on a 12-h/12-h light/dark cycle (light on 07 h-19 h) in a facility that was supervised by veterinarians.
Oocytes were harvested through a mini-laparotomy with frogs anaesthetized, by immersion in water (∼20°C) containing 0.1% tricaine (Sigma-Aldrich, St. Louis, MO, USA). After 15-20 min, the depth of anaesthesia was assessed by pinching the abdomen and lower limbs of the frog with forceps to see if any movement, such as kicking, occurs. The laparotomy was performed only after adequate anaesthesia was attained, based on lack of leg pinch responses. Oocytes were treated with type II collagenase (3.3 mg·mL−1) (Worthington Biochemical, Lakewood, NJ, USA) for 3 h, washed and maintained in ND96 solution (see below for ionic concentrations) supplemented with antibiotics: gentamicin (0.05 mg·mL−1; Invitrogen, Grand Island, NY, USA), amikacin (100 μg·mL−1; Sigma-Aldrich) and ciprofloxacin (2 mg·mL−1; Sigma-Aldrich). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Expression of recombinant GABAA receptors in oocytes
The cDNAs encoding human α1, rat β3, δ, β3-α1-δ trimer and β3-α1 dimer GABAA receptor subunits were generously provided by Dr Erwin Sigel (Department of Biochemistry and Molecular Medicine, University of Bern, Switzerland). The linker between β3 and α1 subunits is composed of 23 amino acids (Q5A3PTGQA3PA2Q5), and that between α1 and δ subunits is 10 amino acids (Q4TGQ4) (Kaur et al., 2009). These subunit cDNAs as well as cDNAs encoding human α1, β3 and γ2L subunits were subcloned into pCMV or pcDNA3.1 vectors. Receptor nomenclature follows BJP's Concise Guide to PHARMACOLOGY (Alexander et al., 2013). mRNA was synthesized using mMESSAGE mMACHINE kits (Ambion, Austin, TX, USA) from linearized cDNA templates, and a poly-A tail was added to mRNA [Poly(A) Tailing Kits; Ambion]. Subunit mRNA concentrations were determined spectrophotometrically. mRNAs mixed at different molar ratios were diluted to desired final concentration (0.01–0.1 ng·nL−1) and microinjected into oocytes (50 nL total). Oocytes were incubated at 18°C in ND96 supplemented with antibiotics until used for electrophysiology (24–72 h).
Two electrode voltage clamp electrophysiology
Oocytes were placed in a low-volume (30 μL) flow chamber continuously perfused by ND96 solution at a rate of 3 mL·min−1. Whole-cell currents were recorded from oocytes using the two electrode voltage clamp technique at room temperature (21–23°C). Recording electrodes were pulled from borosilicate capillary glass (i.d. = 0.68 mm, o.d. = 1.2 mm) (A-M Systems, Sequim, WA, USA). Electrodes were filled with 3 M KCl, and resistance was 1.0–1.4 MΩ.
Oocytes were voltage clamped at −50 or −70 mV (model OC-725C; Warner Instruments, Hamden, CT, USA). Scaled current output was low-pass filtered at 1 kHz, digitized at 100 Hz via a Digidata 1322A interface (Molecular Devices, Sunnyvale, CA, USA) and recorded using Clampex 9.2 software (Molecular Devices). GABA and/or modulator solutions were delivered for 25 s using a custom-built computer-actuated valve controller. A washout interval between consecutive applications ranged from 1 to 5 min depending on the drug concentrations applied. To study the effect of a modulator (ETO, THDOC or zinc) on free or concatenated α1β3δ receptors with different molar ratios and total mRNA amounts, the modulator was pre-applied for 30 s before co-application of GABA (1 mM) and modulator. GABA concentration-response curves were examined in the absence or presence of 3.2 μM ETO for α1β3δ receptors expressed from diluted free or concatenated subunits. ETO was not pre-applied when GABA concentration-response curves were performed. Spontaneous channel activity was investigated by applying a GABAA receptor antagonist picrotoxin (2 mM) in the absence of GABA.
Chemicals and solutions
R-(+)-ETO [2 mg·mL−1 in 35% propylene glycol/water (v v-1) formulation] was obtained from Hospira Inc. (Lake Forest, IL, USA), and other chemicals were purchased from either Sigma-Aldrich or Fisher Scientific (Fair Lawn, NJ, USA), unless otherwise mentioned. ND96 solution was composed of (in mM) 100 NaCl, 2 KCl, 1 CaCl2, 0.8 MgCl2, 1 EGTA and 10 HEPES, pH 7.5. EGTA was omitted from ND96 when ZnCl2 was used in the experiments. GABA (1 M) and ZnCl2 (10 mM) stock solutions were prepared in water, and THDOC stock (10 mM) was prepared in DMSO. Solutions were prepared by diluting the stock solution with ND96 on the day of the experiment. The final concentration of DMSO in experimental solutions was 0.01%. ETO was diluted into ND96. Picrotoxin (2 mM) was dissolved in ND96 by prolonged gentle shaking.
Data analysis
Currents were analysed offline using Clampfit 9.2 (Molecular Devices). Enhancement of saturating GABA (1 mM)-evoked currents by ETO (3.2 μM) or THDOC (1 μM) was determined using the ratio of the peak current elicited by co-application of GABA and ETO (THDOC) and that evoked by GABA alone. For GABA concentration–response data, all currents were normalized to control currents evoked by 1 mM GABA. Normalized concentration–response data were fitted using non-linear least squares with a variable slope logistic equation: 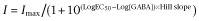 I is the normalized peak current. Imax is the maximal peak current. EC50 is the GABA concentration eliciting 50% of maximal response. Data are reported as mean ± SEM, unless otherwise noted. THDOC, ETO or zinc modulation results in various receptors expressed under different conditions were compared using one-way anova with a post hoc Tukey's multiple comparison test. Statistical significance was inferred if P was less than 0.05.
I is the normalized peak current. Imax is the maximal peak current. EC50 is the GABA concentration eliciting 50% of maximal response. Data are reported as mean ± SEM, unless otherwise noted. THDOC, ETO or zinc modulation results in various receptors expressed under different conditions were compared using one-way anova with a post hoc Tukey's multiple comparison test. Statistical significance was inferred if P was less than 0.05.
Allosteric modelling
Allosteric gating shifts caused by ETO were quantified using a modification of the approach we have previously described for fitting a Monod–Wyman–Changeux (MWC) allosteric co-agonist model (Stewart et al., 2013) to estimated open probability values. Fits were performed using Origin 6.1 (Microcal, Northampton, MA, USA).
Estimated open probability 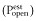 was calculated by explicitly adding average spontaneous activity
was calculated by explicitly adding average spontaneous activity 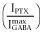 to normalized activated currents
to normalized activated currents 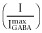 and renormalizing to the full range of open probability, bracketed by maximal ETO-enhanced current (
and renormalizing to the full range of open probability, bracketed by maximal ETO-enhanced current (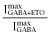 Popen = 1.0) and picrotoxin-blocked basal current (Popen = 0):
Popen = 1.0) and picrotoxin-blocked basal current (Popen = 0):
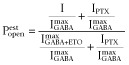 |
1 |
For α1β3δ(1:1:3, 0.5 ng) and β3-α1-δ/β3-α1(1:1, 1 ng) receptors in this study, picrotoxin did not shift basal currents (n = 4 for each receptor), indicating a lack of detectable spontaneous channel activity. Thus, 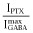 was set at 0. In concentration–response studies of α1β3δ receptors, maximal GABA alone produced responses that were 14- to 20-fold lower than those with GABA plus 3.2 μM ETO. We also estimated maximal Popen in the presence of 3.2 μM ETO by comparing responses to those with GABA + 10 μM ETO, which were assumed to represent response of all activatable receptors (Popen = 1.0) because 30 μM ETO did not further enhance currents. For α1β3δ(1:1:3, 0.5 ng) receptors, 3.2 μM ETO enhanced GABA responses significantly less than 10 μM (n = 6; P < 0.05), and the ratio of
was set at 0. In concentration–response studies of α1β3δ receptors, maximal GABA alone produced responses that were 14- to 20-fold lower than those with GABA plus 3.2 μM ETO. We also estimated maximal Popen in the presence of 3.2 μM ETO by comparing responses to those with GABA + 10 μM ETO, which were assumed to represent response of all activatable receptors (Popen = 1.0) because 30 μM ETO did not further enhance currents. For α1β3δ(1:1:3, 0.5 ng) receptors, 3.2 μM ETO enhanced GABA responses significantly less than 10 μM (n = 6; P < 0.05), and the ratio of 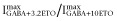 was 0.6 ± 0.11 (mean ± SD). For β3-α1-δ/β3-α1(1:1, 1 ng) receptors, this ratio was 0.76 ± 0.097 (n = 5; P < 0.05). Multiplying these results with ratios for
was 0.6 ± 0.11 (mean ± SD). For β3-α1-δ/β3-α1(1:1, 1 ng) receptors, this ratio was 0.76 ± 0.097 (n = 5; P < 0.05). Multiplying these results with ratios for 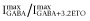 produced GABA efficacy estimates
produced GABA efficacy estimates 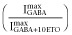 for both free and concatenated α1β3δ receptors ranging from 0.03 to 0.05.
for both free and concatenated α1β3δ receptors ranging from 0.03 to 0.05.
Non-linear least squares fits to the MWC two-state co-agonist mechanism (Equation 2011) used average  data from GABA concentration responses with and without ETO. Both [GABA] and [ETO] are independent continuous variables:
data from GABA concentration responses with and without ETO. Both [GABA] and [ETO] are independent continuous variables:
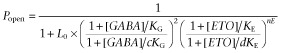 |
2a |
L0 in Equation 2011 is a dimensionless basal equilibrium gating parameter, approximately P0−1. In this study, L0 was set at 25 000 based on previous estimates for α1β2γ2L GABAA receptors (Rusch et al., 2004; Stewart et al., 2013). KG and KE are dissociation constants for GABA and ETO interactions with closed receptors, whereas c and d are single-site efficacy parameters for GABA and ETO respectively. The parameter nE represents the number of ETO sites, usually 2.
We modified the fitting procedure, transforming Equation 2011 into a bimodal function with global parameters for GABA affinity (KG) and efficacy (c), and a single allosteric shift parameter, D, for the ETO concentration we tested:
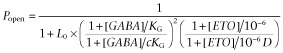 |
2b |
In the modified fitting procedure, [ETO] was treated as a binary variable with a value of 0 when absent and 1 when present. The value of KE = 10−6 was chosen so that in the presence of ETO, [ETO]/KE = 106, mimicking full ETO site occupancy. We also set nE = 1. With these conditions, when ETO is absent, [ETO] = 0, 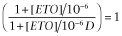 and Equation 2013 describes the concentration response of GABA alone acting at its two agonist sites:
and Equation 2013 describes the concentration response of GABA alone acting at its two agonist sites:
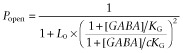 |
3a |
When ETO is present, [ETO] = 1, [ETO]/KE = 106, 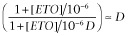 and Equation 2013 becomes:
and Equation 2013 becomes:
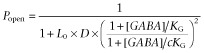 |
3b |
Thus, when  data for GABA concentration-responses in both the absence and presence of ETO are simultaneously fitted using the modified procedure, we obtain estimates (mean ± SEM) for KG, c and D, the latter representing the allosteric shift produced by the experimental ETO concentration. This calculation makes no assumptions about, nor derives estimates for, the affinity, efficacy or number of ETO sites. Its purpose is to quantify allosteric shift by a given modulator concentration, enabling comparisons between receptors where GABA efficacy differs widely, as it does for α1β3γ2 versus α1β3δ receptors.
data for GABA concentration-responses in both the absence and presence of ETO are simultaneously fitted using the modified procedure, we obtain estimates (mean ± SEM) for KG, c and D, the latter representing the allosteric shift produced by the experimental ETO concentration. This calculation makes no assumptions about, nor derives estimates for, the affinity, efficacy or number of ETO sites. Its purpose is to quantify allosteric shift by a given modulator concentration, enabling comparisons between receptors where GABA efficacy differs widely, as it does for α1β3γ2 versus α1β3δ receptors.
Results
Allosteric modulation of α1β3δ GABAA receptors formed from free or concatenated subunits with different molar ratios and total mRNA amounts
We first examined the modulation by THDOC of α1β3 and α1β3δ GABAA receptors formed from free or concatenated subunits with different molar ratios and total mRNA amounts, including α1β3(1:1, 5 ng), α1β3δ(1:1:1, 5 ng), α1β3δ(1:1:1, 0.5 ng), α1β3δ(1:1:3, 5 ng), α1β3δ(1:1:3, 0.5 ng), β3-α1-δ/β3-α1(1:1, 5 ng) and β3-α1-δ/β3-α1(1:1, 1 ng). THDOC at 1 μM potentiated saturating GABA (1 mM)-evoked currents for all the receptors tested (Figure 1 and B). anova analysis (Figure 1B) indicated that THDOC enhancement was not significantly different for receptors expressed with three mRNA mixes at 5 ng per oocyte: α1β3(1:1, 5 ng), 1.6 ± 0.2, (n = 6); α1β3δ(1:1:1, 5 ng), 2.5 ± 0.2, (n = 6); and α1β3δ(1:1:3, 5 ng), 5.3 ± 0.8, (n = 9). Significantly more THDOC potentiation was observed for β3-α1-δ/β3-α1(1:1, 5 ng) receptors (11.9 ± 1.7, n = 9; P < 0.01). Oocytes injected with 0.5 ng α1β3δ(1:1:1, 0.5 ng) mRNAs displayed greater THDOC potentiation (8.6 ± 0.9, n = 6) than that in α1β3(1:1, 5 ng) receptors (P < 0.05), but not statistically different from α1β3δ(1:1:1, 5 ng), α1β3δ(1:1:3, 5 ng) and β3-α1-δ/β3-α1(1:1, 5 ng) receptors. The largest THDOC potentiations were observed in α1β3δ(1:1:3, 0.5 ng) (25.7 ± 2.6, n = 7) and β3-α1-δ/β3-α1(1:1, 1 ng) receptors (23.0 ± 1.0, n = 6), both significantly greater (P < 0.001) than that in α1β3δ(1:1:1, 0.5 ng) receptors (Figure 1B).
Figure 1.
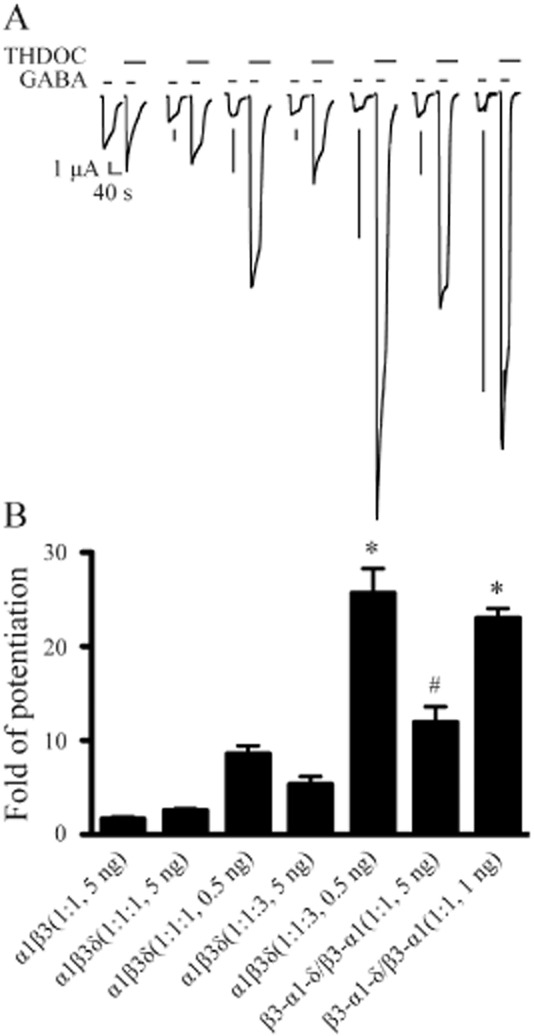
Modulation by THDOC of α1β3δ GABAA receptors expressed from free or concatenated subunits with different molar ratios and total mRNA amounts. (A) Representative current traces evoked by saturating concentration of GABA (1 mM) as well as co-application of 1 mM GABA and 1 μM THDOC with THDOC pre-applied for 30 s. Receptors were expressed by injection of GABAA receptor subunits into oocytes with different molar ratios and total mRNA amounts (see corresponding receptors in panel B). The horizontal bars above each current trace indicate the application of GABA and THDOC respectively. The timescale is the same, and the amplitude scale is 1 μA for all current traces. (B) The fold of potentiation by THDOC for α1β3 receptors as well as α1β3δ receptors formed with different molar ratios and total mRNA amounts injected into oocytes. The error bars represent SEMs. *Significantly different from α1β3δ(1:1:1, 0.5 ng) receptors at P < 0.001. #Significantly different from α1β3(1:1, 5 ng), α1β3δ(1:1:1, 5 ng) and α1β3δ(1:1:3, 5 ng) receptors at P < 0.01 or 0.001.
We next examined potentiation by 3.2 μM ETO in oocytes injected with the same mRNA mixtures tested against THDOC. Allosteric modulation by ETO in this second set of oocytes was similar to that by THDOC (Figure 2 and B). ETO produced significantly greater potentiation in β3-α1-δ/β3-α1(1:1, 5 ng) receptors (14.6 ± 2.0, n = 12) than in α1β3(1:1, 5 ng) (1.7 ± 0.2, n = 8; P < 0.01), α1β3δ(1:1:1, 5 ng) (3.2 ± 0.3, n = 9; P < 0.01) and α1β3δ(1:1:3, 5 ng) receptors (4.4 ± 0.2, n = 9; P < 0.05). Reducing total mRNA injected for α1β3δ receptors (0.5–1.0 ng) also produced more ETO potentiation. ETO potentiation was greatest in α1β3δ(1:1:3, 0.5 ng) (26.4 ± 4.4, n = 9) and β3-α1-δ/β3-α1(1:1, 1 ng) receptors (34.4 ± 3.8, n = 6), and both results were significantly higher than those in α1β3δ(1:1:3, 5 ng), β3-α1-δ/β3-α1(1:1, 5 ng), α1β3δ(1:1:1, 0.5 ng) and α1β3δ(1:1:1, 5 ng) receptors (P < 0.01 or 0.001 for all pairs; Figure 2B).
Figure 2.
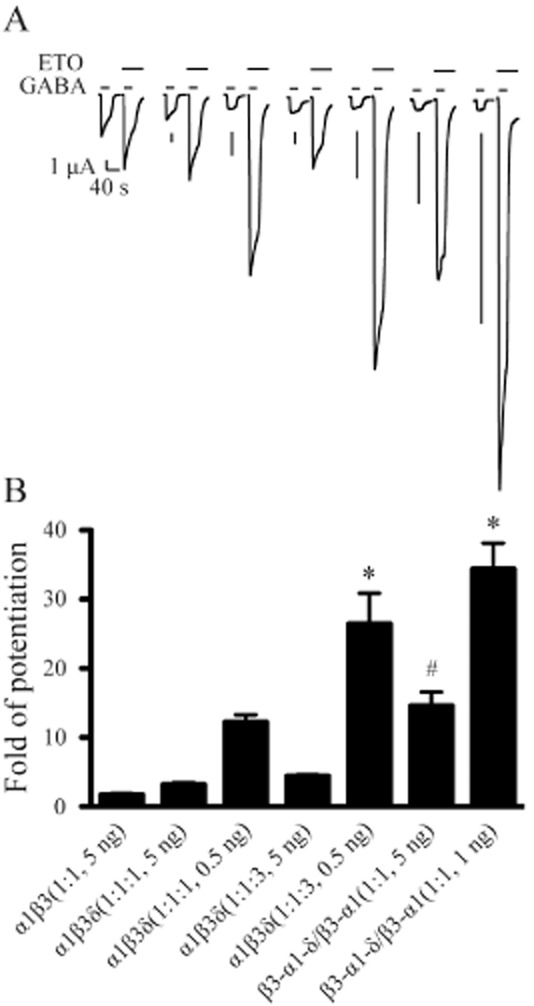
Modulation by ETO of α1β3δ GABAA receptors expressed from free or concatenated subunits with different molar ratios and total mRNA amounts. (A) Representative current traces evoked by saturating concentration of GABA (1 mM) as well as co-application of 1 mM GABA and 3.2 μM ETO with ETO pre-applied for 30 s. Receptors were expressed by injection of GABAA receptor subunits into oocytes with different molar ratios and total mRNA amounts (see corresponding receptors in panel B). The horizontal bars above each current trace indicate the application of GABA and ETO respectively. The timescale is the same, and the amplitude scale is 1 μA for all current traces. (B) The fold of potentiation by ETO for α1β3 receptors as well as α1β3δ receptors formed with different molar ratios and total mRNA amounts injected into oocytes. The error bars represent SEMs. *Significantly different from α1β3δ(1:1:1, 0.5 ng) receptors at P < 0.01 or 0.001. #Significantly different from α1β3(1:1, 5 ng), α1β3δ(1:1:1, 5 ng) and α1β3δ(1:1:3, 5 ng) receptors at P < 0.05 or 0.01.
In addition to the positive modulators, we also examined the effect of zinc, a negative GABAA receptor modulator, on the currents of α1β3(1:1, 5 ng), α1β3δ(1:1:3, 0.5 ng) and β3-α1-δ/β3-α1(1:1, 1 ng) receptors evoked by corresponding EC50 GABA. In line with previous studies in oocytes (Karim et al., 2012; Shu et al., 2012), Zn2+ at 1 μM inhibited GABA (3 μM)-induced currents of α1β3(1:1, 5 ng) receptors by 87.2 ± 1.7% (n = 6). Zn2+ (1 μM) produced significantly less inhibition in both α1β3δ(1:1:3, 0.5 ng) (32.5 ± 4.9%, n = 8; P < 0.001) and β3-α1-δ/β3-α1(1:1, 1 ng) receptors (27.2 ± 4.2%, n = 7; P < 0.001).
ETO produces similar upward and leftward shifts of GABA concentration responses for α1β3δ(1:1:3, 0.5 ng) and β3-α1-δ/β3-α1(1:1, 1 ng) receptors
We examined GABA concentration responses with and without ETO (3.2 μM) in α1β3δ(1:1:3, 0.5 ng), β3-α1-δ/β3-α1(1:1, 1 ng) and α1β3γ2L(1:1:5, 5 ng) receptors in order to compare ETO modulation of these channels quantitatively. The GABA EC50 for α1β3δ(1:1:3, 0.5 ng) receptors was 10.3 μM (n = 4). ETO (3.2 μM) produced an approximately threefold leftward shift (GABA EC50 = 3.7 μM, n = 4), and an ∼17-fold (17.3 ± 2.0) increase in maximal GABA responses (Figure 3). Concatenated β3-α1-δ/β3-α1(1:1, 1 ng) receptors displayed a higher GABA EC50 (74.7 μM, n = 7), consistent with previous reports (Kaur et al., 2009; Baur et al., 2010). In β3-α1-δ/β3-α1(1:1, 1 ng) receptors, ETO produced an approximately threefold leftward shift (GABA EC50 = 22.2 μM) and a large (14.0 ± 2.2-fold) increase in maximal response at high GABA (Figure 4).
Figure 3.
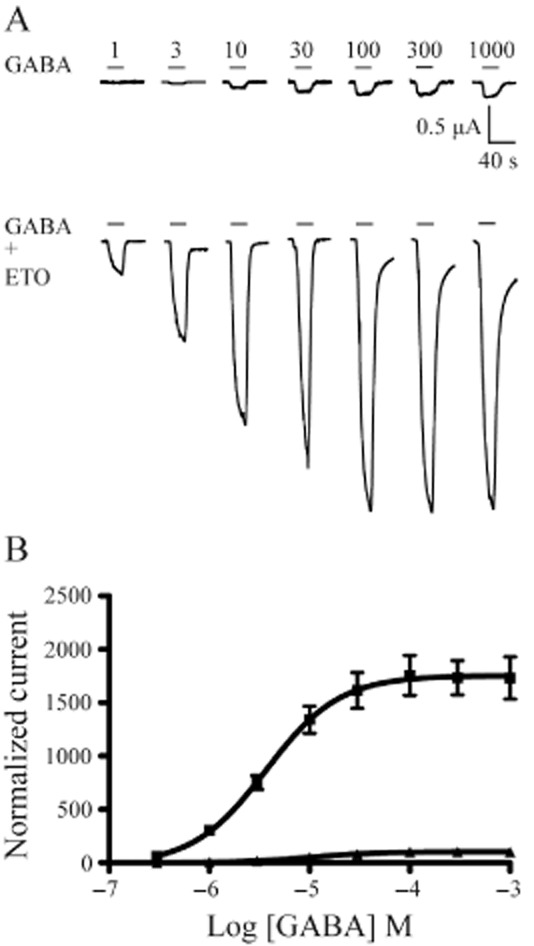
ETO modulation of GABA concentration-response data for α1β3δ(1:1:3, 0.5 ng) receptors. (A) Examples of current traces evoked by increasing concentrations of GABA (in μM) as well as co-application of each concentration of GABA and ETO (3.2 μM) for α1β3δ(1:1:3, 0.5 ng) receptors. (B) The concentration–response curves for GABA alone (triangles) and co-application of GABA with 3.2 μM ETO (squares) were plotted for α1β3δ(1:1:3, 0.5 ng) receptors. ETO produced upward and leftward shifts of the concentration–response curve for the receptors. The horizontal bar above each current trace indicates GABA application or co-application of GABA and ETO. n = 4 cells for GABA or GABA + ETO concentration–response curve. The error bars represent SEMs.
Figure 4.
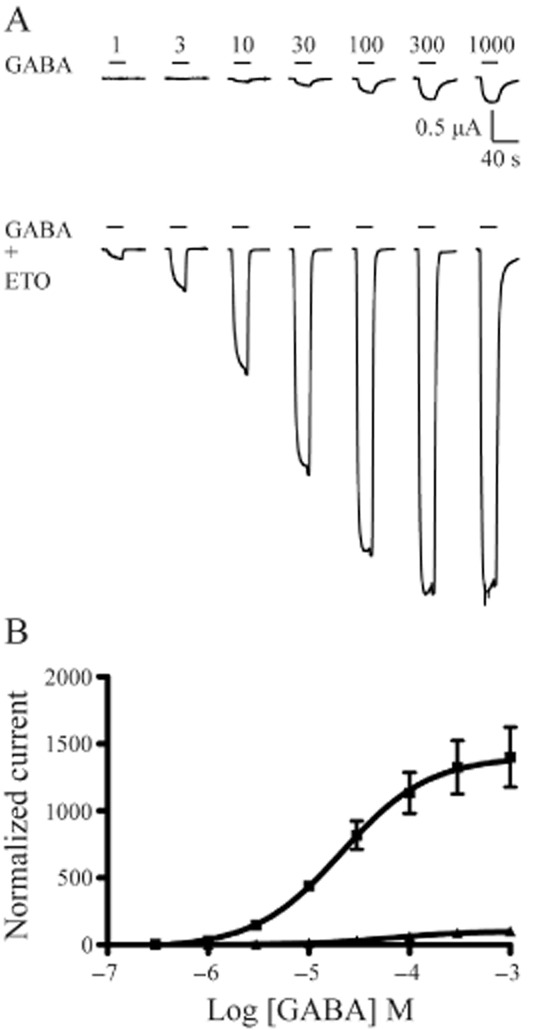
ETO modulation of GABA concentration-response data for β3-α1-δ/β3-α1(1:1, 1 ng) receptors. (A) Examples of current traces evoked by increasing concentrations of GABA (in μM) as well as co-application of each concentration of GABA and ETO (3.2 μM) for β3-α1-δ/β3-α1(1:1, 1 ng) receptors. (B) The concentration–response curves for GABA alone (triangles) and co-application of GABA with 3.2 μM ETO (squares) were plotted for β3-α1-δ/β3-α1(1:1, 1 ng) receptors. ETO produced upward and leftward shifts of the concentration–response curve for the receptors. The horizontal bar above each current trace indicates GABA application or co-application of GABA and ETO. n = 7 cells for GABA or GABA + ETO concentration–response curve. The error bars represent SEMs.
ETO effects on GABA concentration–response parameters for α1β3γ2L receptors differed from those observed in α1β3δ receptors. The GABA EC50 in α1β3γ2L receptors was 7.8 μM (n = 6), and 3.2 μM ETO produced about a 10-fold leftward shift (GABA EC50 = 0.79 μM, n = 7) (Figure 5A). Maximal peak α1β3γ2L receptor currents elicited with 1–3 mM GABA plus ETO were 24% greater than those with GABA alone.
Figure 5.
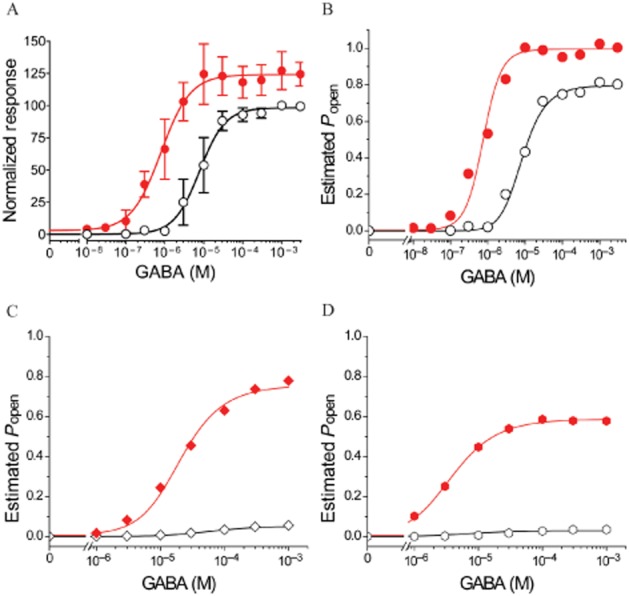
ETO allosteric shift quantified in α1β3γ2L and α1β3δ receptors using MWC co-agonist fits with [ETO] as a binary parameter. (A) Normalized GABA-dependent responses (mean ± SD; n ≥ 5) for α1β3γ2L receptors are plotted. Lines underlying data points are fits to logistic functions. Open symbols are control GABA responses: max = 100 ± 2.5, EC50 = 7.8 (5.0–10.3) μM, nH = 1.3 ± 0.18. Solid red symbols are GABA responses in the presence of 3.2 μM ETO: max = 124 ± 3.0, EC50 = 0.79 (0.55–1.14) μM, nH = 1.2 ± 0.21. (B–D) Estimated Popen was calculated from average data for α1β3γ2L receptors from panel A (B), β3-α1-δ/β3-α1 receptors from Figure 4 (C) and α1β3δ receptors formed from diluted free subunits from Figure 3 (D), as described in Methods section. Simultaneous non-linear least squares fits to Equations 2010 and 2008 were performed as described in Methods section, using [ETO] as a binary parameter (either 0 or 1). (C) Shows β3-α1-δ/β3-α1 receptor results with GABA efficacy = 0.05, and (D) shows α1β3δ receptor results with GABA efficacy = 0.03. The allosteric shifts associated with 3.2 μM ETO are reported in Table 1 along with other fitted parameters.
ETO produces similar allosteric modulation in α1β3δ and α1β3γ2L receptors
We quantified ETO-induced allosteric shifts in GABA concentration responses using a modified global fit procedure and an established model that accounts for allosteric modulation of α1β2γ2L responses to both full and partial agonists (Rusch et al., 2004). In the modified fit procedure, we treated ETO as a binary variable (0 or 1) and collapsed parameters for ETO affinity (KE), efficacy (d) and number of sites (nE) into a single allosteric shift parameter, D (see Equations 2013, 2010 and 2008 in Methods section).
For α1β3γ2L receptors, maximal GABA efficacy was estimated at 0.80. Allosteric shift analysis of α1β3γ2L  values resulted in a good fit (R2 = 0.998) and an allosteric shift factor (D in Equation 2008) of 0.021 (Figure 5; Table 1).
values resulted in a good fit (R2 = 0.998) and an allosteric shift factor (D in Equation 2008) of 0.021 (Figure 5; Table 1).
Table 1.
Fitted MWC parameters2004 for ETO modulation of GABA activation of α1β3γ2L and α1β3δ receptors
| α1β3γ2L | α1β3δ | β3-α1-δ/β3-α1 | |||
|---|---|---|---|---|---|
| GABA efficacy | 0.80 | 0.03 | 0.05 | 0.03 | 0.05 |
| KG (μM) | 10.3 | 3.1 | 7.7 | 14.8 | 25.9 |
| c | 0.0033 | 0.037 | 0.029 | 0.036 | 0.027 |
| D | 0.021 | 0.021 | 0.0072 | 0.035 | 0.018 |
Non-linear least squares fits to Equation 2b were performed as described in Methods section, using [ETO] as a binary parameter (either 0 or 1), with L0 fixed at a value of 25 000, KE fixed at a value of 1 μM and nE = 1. L0 is a dimensionless parameter describing the basal equilibrium between open and closed receptors (O : C). KG is the dissociation constant for GABA binding to closed receptors, whereas c is the efficacy parameter for GABA and D is the allosteric shift produced by 3.2 μM ETO. Maximal GABA efficacy values were based on experimental results (see Methods section) and were used to estimate Popen values before fitting.
For comparison of allosteric shifts in α1β3δ receptors,  was calculated for maximal GABA efficacy values ranging from 0.03 to 0.05. This range is based on both ETO enhancement of responses to maximal GABA alone (Figure 2) and on measurements estimating the efficacy of maximal GABA plus 3.2 μM ETO (by comparison with GABA plus 10 μM ETO). Allosteric shift parameters for α1β3δ(1:1:3, 0.5 ng) receptors ranged from 0.0072 to 0.021, and the shift parameters for β3-α1-δ/β3-α1(1:1, 1 ng) receptors ranged from 0.018 to 0.035 (Figure 5 and D; Table 1).
was calculated for maximal GABA efficacy values ranging from 0.03 to 0.05. This range is based on both ETO enhancement of responses to maximal GABA alone (Figure 2) and on measurements estimating the efficacy of maximal GABA plus 3.2 μM ETO (by comparison with GABA plus 10 μM ETO). Allosteric shift parameters for α1β3δ(1:1:3, 0.5 ng) receptors ranged from 0.0072 to 0.021, and the shift parameters for β3-α1-δ/β3-α1(1:1, 1 ng) receptors ranged from 0.018 to 0.035 (Figure 5 and D; Table 1).
Discussion
Although αβδ GABAA receptors are recognized as important mediators of neurosteroid modulation and general anaesthetic actions (Belelli et al., 2009), no consensus has emerged on the pentameric arrangement of α, β and δ subunits formed from heterologously expressed free subunits, or that in neurons. Expression of concatenated assemblies of GABAA receptor subunits was instrumental in defining the assembly of αβγ receptors (Baumann et al., 2001) and has been previously applied to αβδ (Kaur et al., 2009; Sigel et al., 2009; Baur et al., 2010; Shu et al., 2012). However, several issues contribute to a lack of clarity emerging from these studies. First, as discussed below, oocyte expression of free α, β and δ subunits, more so than for αβγ, apparently results in different subunit arrangements, depending on the subunit subtypes, the mRNA ratio and, as we demonstrate, the total amount of mRNA injected. Thus, the configuration and functional properties of ‘control’ receptors formed from free subunits may vary among studies, or even within a single study. Second, in most previous studies, comparison of receptors has been based on both GABA sensitivity and neurosteroid modulation. Subunit concatenation is associated with increased GABA EC50s in both αβγ (Baumann et al., 2002; Steinbach and Akk, 2011) and αβδ receptors (Kaur et al., 2009; Baur et al., 2010), a result we also observed in the current study, making this functional parameter an unreliable comparator. In addition, comparing neurosteroid modulation remains largely empirical because the number and location of neurosteroid sites remain unknown, nor do we know if subunit concatenation affects steroid modulation. We have addressed these issues in two ways. First, we used a criterion of maximized modulator (THDOC and ETO) effects to determine conditions for optimal α1β3δ receptor expression in oocytes. Second, to strengthen inferences about the configuration of subunits in α1β3δ receptors, we quantified the effects of R-(+)-ETO, a potent stereoselective allosteric modulator that in αβγ receptors acts selectively at sites formed between transmembrane α-M1 and β-M3 domains. Importantly, concatenation in α1β2γ2L receptors does not significantly affect ETO modulation (Guitchounts et al., 2012).
Function of oocyte-expressed α1β3δ receptors depends on subunit mRNA molar ratios and total amount injected
Previous studies have demonstrated that many GABAA receptor modulators, including THDOC, potentiate maximal GABA-elicited currents in α1βδ receptors far more than those in α1β receptors (Wohlfarth et al., 2002; Feng and Macdonald, 2004; 2010,; Zheleznova et al., 2008; Lewis et al., 2010). These observations indicate that GABA is a weak partial agonist of δ subunit-containing receptors. However, others have reported little difference in drug (including ETO) modulation of maximal GABA responses in α4β versus α4βδ receptors (Meera et al., 2009; Lewis et al., 2010), suggesting variable δ incorporation and/or that α subunits also play an important role in αβδ receptor modulation (Jensen et al., 2013). In our current study, we varied subunit mRNA ratios and total amount injected, and found that the resulting patterns of modulation by both THDOC and ETO were similar (Figures 1 and 2). Enhancement of maximal GABA currents in oocytes injected with 5 ng total mRNA encoding free α1, β3 and δ subunits was similar to that in oocytes injected with mRNAs for α1 and β3. The most likely interpretation, in agreement with others (Borghese and Harris, 2007; Karim et al., 2012; Shu et al., 2012), is that δ subunits do not efficiently incorporate into oocyte-expressed receptors under these conditions, which we have used successfully to express αβγ receptors.
At 5 ng total mRNA per oocyte, increasing the ratio of δ mRNA threefold relative to α and β did not significantly affect modulator efficacy in our study. However, concatenated β3-α1-δ/β3-α1(1:1) mRNA resulted in greater THDOC and ETO potentiation than α1β3δ(1:1:1) or α1β3δ(1:1:3), indicating the expected incorporation of δ as reported by others (Kaur et al., 2009; Shu et al., 2012). We also observed that expression of either β-α (dimer) or β-α-δ (trimer) alone results in surface receptors that are activated by GABA, as reported previously (Kaur et al., 2009). It is possible that three dimers or two trimers form pentamers with one extra subunit appended, as demonstrated in nicotinic ACh receptors (Zhou et al., 2003; Minier and Sigel, 2004). However, the functional properties of receptors formed from co-expression of dimers and trimers are reportedly similar to those of fully concatenated pentameric receptors (Kaur et al., 2009; Sigel et al., 2009), suggesting that when both dimers and trimers are present, pentamers are preferentially formed from one of each. Our gating modulation results also indicate that free subunit α1β3δ receptors best match those formed from the co-expression of concatenated dimers and trimers together.
Unexpectedly, we found that injecting 10-fold less mRNA (0.5 ng) at 1α:1β:3δ mRNA ratio markedly increased THDOC and ETO modulation (and also reduced zinc inhibition). With lower total mRNA, free subunit mRNA ratios also affected modulation; α1β3δ (1:1:3, 0.5 ng) receptors displayed more modulation than a 1:1:1 mix. Thus, subunit mRNA ratio apparently affects the stoichiometry and configuration of oocyte surface receptors, consistent with other studies varying the α:β:δ ratios of mRNA in oocytes (Shu et al., 2012) or cDNAs transfected into HEK cells (Botzolakis et al., 2008; Wagoner and Czajkowski, 2010). Reducing total mRNA also enhanced modulation of oocyte-expressed receptors formed from β3-α1-δ and β3-α1 concatemers, although less so than with free subunits. Our novel observation that total mRNA affects surface receptor function (and presumably structure) suggests that competition among subunit mRNAs for limited translation capacity, assembly and/or trafficking elements in oocytes may contribute to variable receptor assembly. Further studies are needed to explore these potential mechanisms.
ETO produces similar allosteric modulation for α1β3δ and α1β3γ2L GABAA receptors
Comparison of ETO effects on GABA-dependent responses in α1β3δ(1:1:3, 0.5 ng) and β3-α1-δ/β3-α1(1:1, 1 ng) receptors demonstrated similar reductions in GABA EC50 and increases in maximal responses for both receptors. The effects of ETO on GABA-dependent α1β3γ2L currents appear very different from those in α1β3δ receptors, but similar to previous results in α1β2γ2L receptors formed with both free (Rusch et al., 2004) and concatenated subunits (Guitchounts et al., 2012).
ETO's allosteric effects were also quantified based on a MWC mechanism that accounts for modulation of α1β2γ2 currents elicited with both full and partial agonists (Rusch et al., 2004; Forman, 2012). Allosteric shift analysis of α1β3γ2L data indicates that 3.2 μM ETO shifts the closed-open equilibrium 48-fold towards open, or about sevenfold for each ETO site. In comparison, β3-α1-δ/β3-α1 data indicate a 29- to 55-fold shift in the closed-open equilibrium. Allosteric shifts in free subunit α1β3δ receptors (48- to 140-fold) overlap with the values for β3-α1-δ/β3-α1 and α1β3γ2L receptors. The similarity of ETO allosteric shifts contrasts with the dramatic difference in GABA efficacy for αβγ and αβδ receptors. The similar allosteric shifts are consistent with the presence of two α1-M1/β3-M3 inter-subunit ETO sites on each of these receptors, favouring the hypothesis that the subunit arrangement of α1β3δ receptors is β3-α1-δ-β3-α1, similar to α1β3γ2L.
Our inferences regarding the arrangement of α1β3δ subunits are not conclusive. The range of allosteric shift estimates for α1β3δ reflects the uncertain gating efficacy of GABA, which appears to be a very weak partial agonist in these receptors (Table 1). Nonetheless, these estimates are consistent with previous reports that neurosteroids increase maximal GABA responses in oocyte-expressed α1β2/3δ by 20-fold or more (Zheleznova et al., 2008; Kaur et al., 2009). Given the low efficacy of GABA, we expected the fitted MWC model resting state dissociation constant, KG, to be close to EC50. However, fitted KG values (Table 1) are consistently lower than GABA EC50 for α1β3δ (Figures 3 and 4). This discrepancy decreased as GABA efficacy was increased during analysis. Alternative structural hypotheses may also be consistent with our results. ETO activates β3 homo-oligomeric receptors (Cestari et al., 1996) and photolabel derivatives apparently bind at β3-M1/β3-M3 transmembrane interfaces (Chiara et al., 2012). Thus, configurations of 2α1, 2β3 and 1δ that form β3-M1/β3-M3 interfaces might be modulated similarly to those with two α1-M1/β3-M3 sites. It is also conceivable, given that δ is phylogenetically closer to β than to γ2, that δ may form ETO sites with adjacent α or β subunits. Combining concatenated subunit assemblies with binding site mutations known to alter ETO sensitivity will be informative in testing these alternative structures.
Conclusions
In Xenopus oocytes, functional expression of cell surface α1β3δ GABAA receptors is influenced both by the ratio of subunit mRNAs and by the total amount of mRNA. Surprisingly, reducing total mRNA promotes the incorporation of δ subunits into receptors. Concatenated αβδ subunit assemblies also enhance the incorporation of δ. Analysis using an established allosteric model showed that ETO has quantitatively similar modulatory effects in αβδ and αβγ receptors. Our results favour the hypothesis that the arrangements of α1β3δ and α1β3γ2L subunits are similar.
Acknowledgments
We thank Mayo Hotta for preliminary studies using reduced mRNA injection and Dr Kunpeng Liu for helpful discussion. This work was supported by P01GM058448 and R01GM089745 to S. A. F.
Glossary
- ETO
etomidate
- MWC
Monod–Wyman–Changeux
- THDOC
tetrahydrodeoxycorticosterone
Conflict of interest
None.
References
- Akk G, Steinbach JH. Structural studies of the actions of anesthetic drugs on the γ-aminobutyric acid type A receptor. Anesthesiology. 2011;115:1338–1348. doi: 10.1097/ALN.0b013e3182315d93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Bracamontes JR, Covey DF, Evers A, Dao T, Steinbach JH. Neuroactive steroids have multiple actions to potentiate GABAA receptors. J Physiol. 2004;558:59–74. doi: 10.1113/jphysiol.2004.066571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, et al. The Concise Guide to PHARMACOLOGY 2013/14: Overview. Br J Pharmacol. 2013;170:1449–1867. doi: 10.1111/bph.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C, Sturt BL, Bamber BA. Multiple roles for the first transmembrane domain of GABAA receptor subunits in neurosteroid modulation and spontaneous channel activity. Neurosci Lett. 2010;473:242–247. doi: 10.1016/j.neulet.2010.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera NP, Betts J, You H, Henderson RM, Martin IL, Dunn SM, et al. Atomic force microscopy reveals the stoichiometry and subunit arrangement of the α4β3δ GABAA receptor. Mol Pharmacol. 2008;73:960–967. doi: 10.1124/mol.107.042481. [DOI] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. Subunit arrangement of γ-aminobutyric acid type A receptors. J Biol Chem. 2001;276:36275–36280. doi: 10.1074/jbc.M105240200. [DOI] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. Forced subunit assembly in α1β2γ2 GABAA receptors. Insight into the absolute arrangement. J Biol Chem. 2002;277:46020–46025. doi: 10.1074/jbc.M207663200. [DOI] [PubMed] [Google Scholar]
- Baur R, Kaur KH, Sigel E. Diversity of structure and function of α1α6β3δ GABAA receptors: comparison with α1β3δ and α6β3δ receptors. J Biol Chem. 2010;285:17398–17405. doi: 10.1074/jbc.M110.108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese CM, Harris RA. Studies of ethanol actions on recombinant δ-containing γ-aminobutyric acid type A receptors yield contradictory results. Alcohol. 2007;41:155–162. doi: 10.1016/j.alcohol.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botzolakis EJ, Stanic AK, Gurba KN, Lagrange AH, Feng HJ, Hu NN, et al. Flow cytometric analysis of GABAA receptor surface expression: evidence that αβγ and αβδ isoforms have similar subunit stoichiometries and arrangements. Soc Neurosci Abstr. 2008;34:427.14. [Google Scholar]
- Bracamontes J, McCollum M, Esch C, Li P, Ann J, Steinbach JH, et al. Occupation of either site for the neurosteroid allopregnanolone potentiates the opening of the GABAA receptor induced from either transmitter binding site. Mol Pharmacol. 2011;80:79–86. doi: 10.1124/mol.111.071662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cestari IN, Uchida I, Li L, Burt D, Yang J. The agonistic action of pentobarbital on GABAA β-subunit homomeric receptors. Neuroreport. 1996;7:943–947. doi: 10.1097/00001756-199603220-00023. [DOI] [PubMed] [Google Scholar]
- Chang Y, Wang R, Barot S, Weiss DS. Stoichiometry of a recombinant GABAA receptor. J Neurosci. 1996;16:5415–5424. doi: 10.1523/JNEUROSCI.16-17-05415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiara DC, Dostalova Z, Jayakar SS, Zhou X, Miller KW, Cohen JB. Mapping general anesthetic binding site(s) in human α1β3 γ-aminobutyric acid type A receptors with [3H]TDBzl-etomidate, a photoreactive etomidate analogue. Biochemistry. 2012;51:836–847. doi: 10.1021/bi201772m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Feng HJ. Allosteric modulation of αβδ GABAA receptors. Pharmaceuticals. 2010;3:3461–3477. Available at: http://www.mdpi.com/journal/pharmaceuticals (accessed 11/3/2010) [Google Scholar]
- Feng HJ, Macdonald RL. Proton modulation of α1β3δ GABAA receptor channel gating and desensitization. J Neurophysiol. 2004;92:1577–1585. doi: 10.1152/jn.00285.2004. [DOI] [PubMed] [Google Scholar]
- Feng HJ, Macdonald RL. Barbiturates require the N terminus and first transmembrane domain of the δ subunit for enhancement of α1β3δ GABAA receptor currents. J Biol Chem. 2010;285:23614–23621. doi: 10.1074/jbc.M110.122564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SA. Monod–Wyman–Changeux allosteric mechanisms of action and the pharmacology of etomidate. Curr Opin Anaesthesiol. 2012;25:411–418. doi: 10.1097/ACO.0b013e328354feea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SA, Miller KW. Anesthetic sites and allosteric mechanisms of action on Cys-loop ligand-gated ion channels. Can J Anaesth. 2011;58:191–205. doi: 10.1007/s12630-010-9419-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitchounts G, Stewart DS, Forman SA. Two etomidate sites in α1β2γ2 γ-aminobutyric acid type A receptors contribute equally and noncooperatively to modulation of channel gating. Anesthesiology. 2012;116:1235–1244. doi: 10.1097/ALN.0b013e3182567df3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevers W, Luddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Jensen ML, Wafford KA, Brown AR, Belelli D, Lambert JJ, Mirza NR. A study of subunit selectivity, mechanism and site of action of the δ selective compound 2 (DS2) at human recombinant and rodent native GABAA receptors. Br J Pharmacol. 2013;168:1118–1132. doi: 10.1111/bph.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim N, Wellendorph P, Absalom N, Bang LH, Jensen ML, Hansen MM, et al. Low nanomolar GABA effects at extrasynaptic α4β1/β3δ GABAA receptor subtypes indicate a different binding mode for GABA at these receptors. Biochem Pharmacol. 2012;84:549–557. doi: 10.1016/j.bcp.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Kaur KH, Baur R, Siegel E. Unanticipated structural and functional properties of δ-subunit-containing GABAA receptors. J Biol Chem. 2009;284:7889–7896. doi: 10.1074/jbc.M806484200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RW, Mabry J, Polisar JG, Eagen KP, Ganem B, Hess GP. Dihydropyrimidinone positive modulation of δ-subunit-containing γ-aminobutyric acid type A receptors, including an epilepsy-linked mutant variant. Biochemistry. 2010;49:4841–4851. doi: 10.1021/bi100119t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GD, Chiara DC, Sawyer GW, Husain SS, Olsen RW, Cohen JB. Identification of a GABAA receptor anesthetic binding site at subunit interfaces by photolabeling with an etomidate analog. J Neurosci. 2006;26:11599–11605. doi: 10.1523/JNEUROSCI.3467-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Meera P, Olsen RW, Otis TS, Wallner M. Etomidate, propofol and neurosteroid THDOC increase the GABA efficacy of recombinant α4β3δ and α4β3 GABAA receptors expressed in HEK cells. Neuropharmacology. 2009;56:155–160. doi: 10.1016/j.neuropharm.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minier F, Sigel E. Techniques: use of concatenated subunits for the study of ligand-gated ion channels. Trends Pharmacol Sci. 2004;25:499–503. doi: 10.1016/j.tips.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABAA receptors. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch D, Zhong H, Forman SA. Gating allosterism at a single class of etomidate sites on α1β2γ2L GABAA receptors accounts for both direct activation and agonist modulation. J Biol Chem. 2004;279:20982–20992. doi: 10.1074/jbc.M400472200. [DOI] [PubMed] [Google Scholar]
- Shu HJ, Bracamontes J, Taylor A, Wu K, Eaton MM, Akk G, et al. Characteristics of concatemeric GABAA receptors containing α4/δ subunits expressed in Xenopus oocytes. Br J Pharmacol. 2012;165:2228–2243. doi: 10.1111/j.1476-5381.2011.01690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel E, Kaur KH, Luscher BP, Baur R. Use of concatamers to study GABAA receptor architecture and function: application to δ-subunit-containing receptors and possible pitfalls. Biochem Soc Trans. 2009;37:1338–1342. doi: 10.1042/BST0371338. [DOI] [PubMed] [Google Scholar]
- Steinbach JH, Akk G. Use of concatemers of ligand-gated ion channel subunits to study mechanisms of steroid potentiation. Anesthesiology. 2011;115:1328–1337. doi: 10.1097/ALN.0b013e318233046a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DS, Hotta M, Desai R, Forman SA. State-dependent etomidate occupancy of its allosteric agonist sites measured in a cysteine-substituted GABAA receptor. Mol Pharmacol. 2013;83:1200–1208. doi: 10.1124/mol.112.084558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Ehya N, Fuchs K, Sieghart W. Stoichiometry and assembly of a recombinant GABAA receptor subtype. J Neurosci. 1997;17:2728–2737. doi: 10.1523/JNEUROSCI.17-08-02728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagoner KR, Czajkowski C. Stoichiometry of expressed α4β2δ γ-aminobutyric acid type A receptors depends on the ratio of subunit cDNA transfected. J Biol Chem. 2010;285:14187–14194. doi: 10.1074/jbc.M110.104257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances α4β3δ and α6β3δ γ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci U S A. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABAA receptors containing the δ subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheleznova N, Sedelnikova A, Weiss DS. α1β2δ, a silent GABAA receptor: recruitment by tracazolate and neurosteroids. Br J Pharmacol. 2008;153:1062–1071. doi: 10.1038/sj.bjp.0707665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Nelson ME, Kuryatov A, Choi C, Cooper J, Lindstrom J. Human α4β2 acetylcholine receptors formed from linked subunits. J Neurosci. 2003;23:9004–9015. doi: 10.1523/JNEUROSCI.23-27-09004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


