Table 1. Gold-Catalyzed Nazarov Cyclizations Aryl Enone 1a.
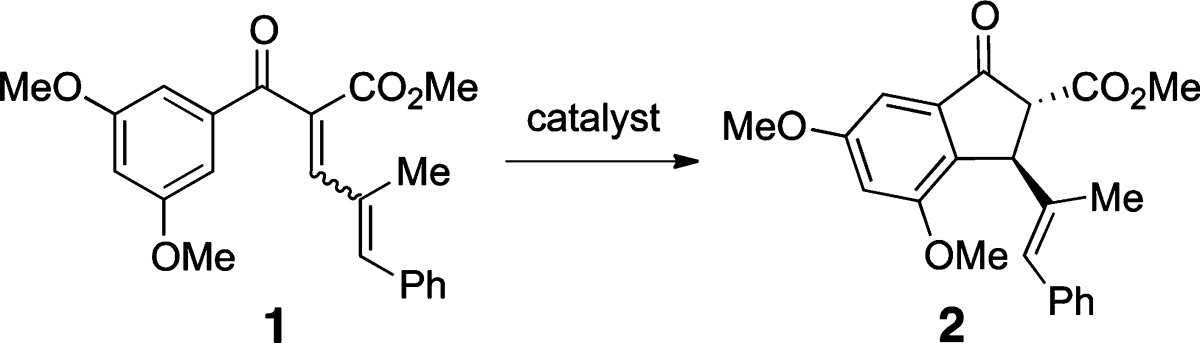
| entry | catalyst | temp (°C) | time (h) | yield (%)b |
|---|---|---|---|---|
| 1 | AuCl3/AgSbF6 (1:3) | rt | 0.5 | >99 |
| 2 | AuCl3/AgSbF6 (1:2) | rt | 0.5 | >99 |
| 3 | AuCl3/AgSbF6 (1:1) | rt | 0.5 | –d |
| 4 | AuCl3/AgSbF6 (1:2) recycled once | rt | 0.5 | >99 |
| 5 | AuCl3/AgSbF6 (1:2) recycled twice | rt | 0.5 | 97 |
| 6 | (AuCl)2dppe/AgSbF6 | rt | 24 | NR |
| 7 | AuCl3/Na[BArF4] (1:2) | rt | 0.5 | >99 |
| 8 | AuCl3/AgSbF6 (1:2) + Proton-spongee | rt | 1 | >99 |
| 9 | AuCl3c | 60 | 24 | –d |
| 10 | AgSbF6c | 60 | 24 | –d |
Reaction conditions: 1 mol % of AuCl3 was used; catalyst was premixed for 0.5 h prior to reaction in dichloromethane or toluene; reactions containing AgSbF6 were run under minimal light conditions.
Isolated yields.
15 mol % was used.
Incomplete reactions monitored by thin layer chromatography (<50% conversion).
100 mol % was used. NR = no reaction, dppe =1,2-bis(diphenylphosphino)ethane, [BArF4]− = tetrakis[3,5-bis(trifluoromethyl)phenyl]borate, Proton-sponge =1,8-bis(dimethylamino)naphthalene.
