Table 3. Nazarov/Lactonization Cascade Reactions of Aryl Vinyl Enones with Heterogeneous Gold Catalysta.
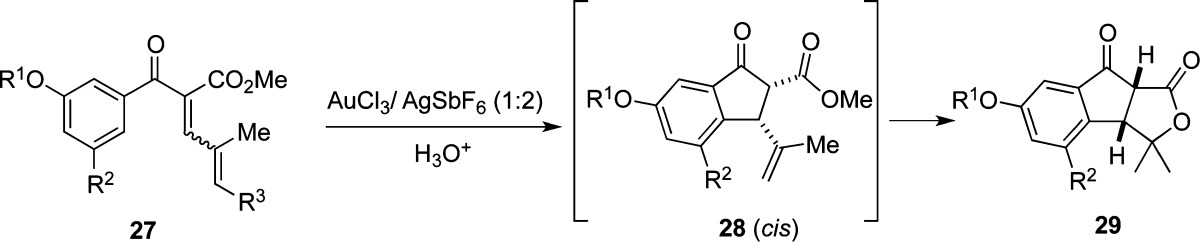
| no. | dienone | R1 | R2 | R3 | timeb (h) | temp (°C) | solvent | additive | product | yieldc (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27a | TIPS | Br | TBS | 5 | rt | CH2Cl2 | none | 29a | 79 |
| 2 | 27b | TIPS | Br | TMS | 20 | 60 | CH2Cl2/PhMe (1:1) | none | 29a | 82 |
| 3 | 27c | Me | OMe | TBS | 24 | 60 | CH2Cl2/PhMe (1:1) | none | 29b | 83 |
| 4 | 27d | Me | H | TMS | 24 | 60 | CH2Cl2/PhMe (1:1) | none | 29c | 88 |
| 5 | 27c | Me | OMe | TBS | 24 | 60 | CH2Cl2/PhMe (1:1) | Proton-sponged | – | NR |
| 6 | 27c | Me | OMe | TBS | 24 | 60 | CH2Cl2/PhMe (1:1) | 3 Å mol sieves | 28b | 80 |
| 7 | 27c | Me | OMe | TBS | 24 | 60 | CH2Cl2/PhMe (1:1) | HOTf (1 equiv)e | 27e (R3=H) | 92 |
Reaction conditions (unless otherwise specified): 1 mol % of AuCl3 was used; catalyst was premixed prior to reaction; reactions were protected from light.
Reactions monitored using thin layer chromatography.
Isolated yields.
100 mol %.
Reaction was conducted without the AuCl3/AgSbF6 catalyst. TBS = tert-butyldimethylsilyl, TIPS = triisopropylsilyl, TMS = trimethylsilyl, Proton-sponge = 1,8-bis(dimethylamino)naphthalene. NR = no reaction, HOTf = triflic acid.
