Table 3. Benzothiazoles Derivatives Synthesized and Evaluated as CK-1δ Inhibitors.
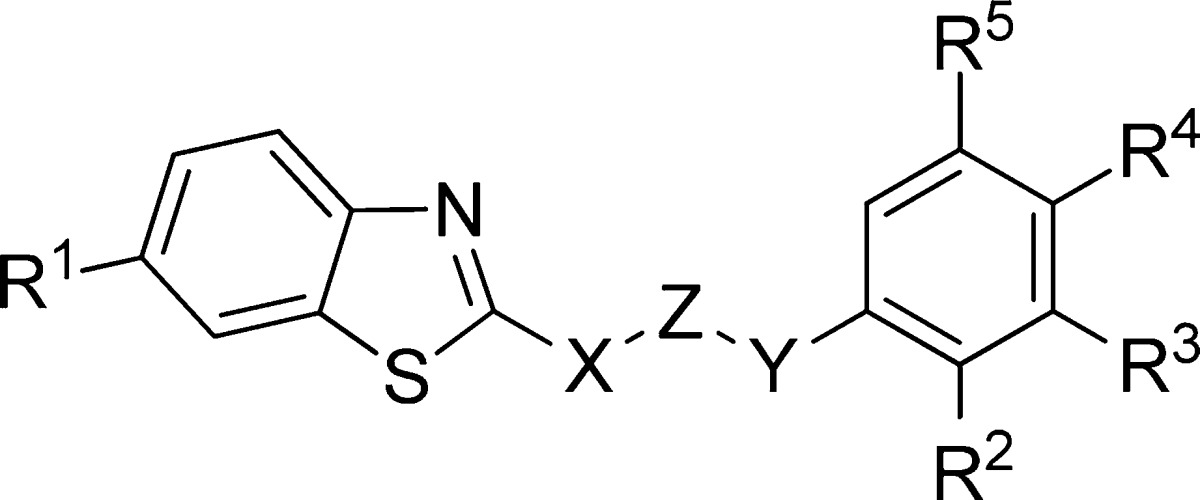
| compd | X | Z | Y | R1 | R2 | R3 | R4 | %inh @10 μM (%) | CK-1δ IC50 (μM) |
|---|---|---|---|---|---|---|---|---|---|
| MR-3.15 | NH | CO | CH2 | H | H | Cl | H | >60 | 0.85 ± 0.10 |
| 9 | O | CO | CH2 | H | H | H | Cl | 20 | |
| 10 | NH | CO | CF3 | OMe | H | H | 25 | ||
| 11 | NH | CH2 | CH2 | CF3 | OMe | H | H | 25 | |
| 12 | NH | CO | CHPh | H | H | H | H | >60 | 1.96 ± 0.83 |
| 13 | NH | CO | CHPh | OEt | H | H | H | >60 | 2.82 ± 0.43 |
| 14 | NH | CO | CH2CH2 | H | H | Cl | H | >60 | 3.58 ± 0.21 |
| 15 | NH | CO | CH2CHPh | H | H | H | H | >60 | 2.50 ± 0.33 |
| 16 | NH | CO | NH | CF3 | H | H | OMe | >60 | 5.50 ± 0.11 |
