Table 4. N-Benzothiazolyl-2-phenyl-acetamides Derivatives Synthesized and Evaluated as CK-1δ Inhibitors.
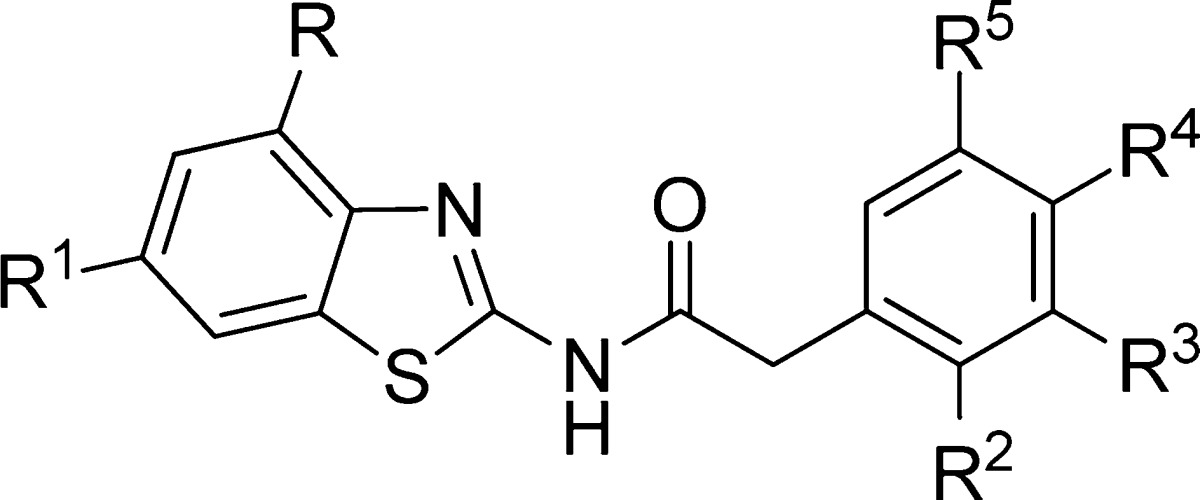
| compd | R | R1 | R2 | R3 | R4 | R5 | %inh @10 μM (%) | CK-1δ IC50 (μM) |
|---|---|---|---|---|---|---|---|---|
| MR-3.15 | H | H | H | Cl | H | H | >60 | 0.85 ± 0.10 |
| 17 | H | Me | H | Cl | H | H | >60 | 0.083 ± 0.003 |
| 18 | Me | H | H | Cl | H | H | 20 | |
| 19 | Cl | H | H | Cl | H | H | 20 | |
| 20 | H | CF3 | H | Cl | H | H | >60 | 0.023 ± 0.002 |
| 21 | H | OMe | H | Cl | H | H | >60 | 0.53 ± 0.07 |
| 22 | H | OCF3 | H | Cl | H | H | >60 | 0.54 ± 0.02 |
| 23 | H | OEt | H | Cl | H | H | >60 | 1.21 ± 0.09 |
| 24 | H | CF3 | Cl | H | H | H | >60 | 0.068 ± 0.007 |
| 25 | H | OMe | Cl | H | H | H | >60 | 9.71 ± 0.99 |
| 26 | H | OEt | Cl | H | H | H | >60 | 17.43 ± 1.21 |
| 27 | H | CF3 | H | H | Cl | H | >60 | 0.065 ± 0.003 |
| 28 | H | OMe | H | H | Cl | H | >60 | 0.75 ± 0.09 |
| 29 | H | OEt | H | H | Cl | H | >60 | 1.11 ± 0.29 |
| 30 | H | Br | OMe | H | H | H | >60 | 0.26 ± 0.02 |
| 31 | H | Cl | OMe | H | H | H | >60 | 0.32 ± 0.03 |
| 32 | H | F | OMe | H | H | H | >60 | 1.17 ± 0.51 |
| 33 | H | Me | OMe | H | H | H | >60 | 0.29 ± 0.03 |
| 34 | H | CF3 | OMe | H | H | H | >60 | 0.010 ± 0.001 |
| 35 | H | OMe | OMe | H | H | H | >60 | 2.22 ± 0.30 |
| 36 | H | OCF3 | OMe | H | H | H | >60 | 0.62 ± 0.06 |
| 37 | H | OEt | OMe | H | H | H | >60 | 5.76 ± 0.61 |
| 38 | H | CF3 | H | OMe | H | H | >60 | 0.042 ± 0.050 |
| 39 | H | OMe | H | OMe | H | H | >60 | 0.42 ± 0.06 |
| 40 | H | OEt | H | OMe | H | H | >60 | 0.99 ± 0.07 |
| 41 | H | CF3 | H | CF3 | H | H | >60 | 0.087 ± 0.033 |
| 42 | H | CF3 | H | H | OMe | H | >60 | 0.033 ± 0.002 |
| 43 | H | OMe | H | H | OMe | H | >60 | 0.57 ± 0.08 |
| 44 | H | OEt | H | H | OMe | H | >60 | 1.09 ± 0.12 |
| 45 | H | H | H | H | H | H | >60 | 0.33 ± 0.03 |
| 46 | H | CF3 | H | H | H | H | >60 | 0.047 ± 0.005 |
| 47 | H | CF3 | H | Cl | Cl | H | >60 | 0.056 ± 0.002 |
| 48 | H | OMe | H | Cl | Cl | H | >60 | 1.24 ± 0.17 |
| 49 | H | OEt | H | Cl | Cl | H | >60 | 3.43 ± 0.69 |
| 50 | H | OCF3 | H | Cl | Cl | H | >60 | 0.59 ± 0.04 |
| 51 | H | CF3 | OMe | H | H | OMe | >60 | 0.19 ± 0.05 |
| 52 | H | OCF3 | H | OMe | OMe | OMe | >60 | 0.079 ± 0.007 |
| 53 | H | OMe | H | OMe | OMe | OMe | >60 | 1.12 ± 0.41 |
| 54 | H | OEt | H | OMe | OMe | OMe | >60 | 1.43 ± 0.35 |
| 55 | H | CF3 | H | OMe | OMe | OMe | >60 | 0.015 ± 0.007 |
