Figure 1.
Expression of Moderate Levels of Nondegradable Cyclin B1 Separates APC/CCdc20 Activation and Sister Chromatid Disjunction from Cdk1 Inactivation
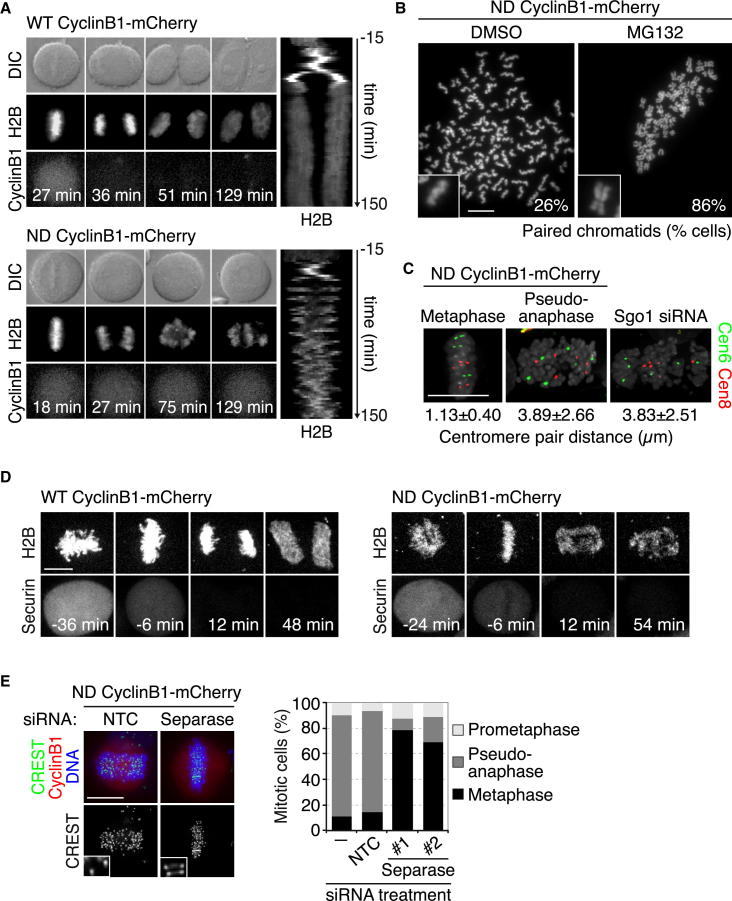
(A) Time-lapse series and kymograph of H2B-EGFP HeLa cells expressing wild-type (WT) or nondegradable (ND) cyclin B1-mCherry. While 93% of cells expressing ND cyclin B1 arrested in mitosis, all cells expressing WT cyclin B1 exited mitosis (n > 40 cells from three independent experiments). The first time frame after nuclear envelope breakdown (NEBD) corresponds to t = 0 min. See also Figure S1.
(B) Chromosome spreads of cells expressing ND cyclin B1 that were synchronously released into mitosis and treated with DMSO (control) or the proteasome inhibitor MG132 (10 μM) for 4 hr (n > 299 spreads from 3 independent experiments). See also Figures S1E and S1F.
(C) Fluorescence in situ hybridization (FISH) analysis of the centromeres of trisomic chromosomes 6 and 8. Distance between the closest centromeres was measured on 3D pictures (n > 29 cells). Depletion of Sgo1 was used as a control for loss of sister chromatid cohesion.
(D) Analysis of securin degradation. Confocal live-cell imaging of H2B-iRFP HeLa cells expressing securin-EGFP and WT or ND cyclin B1-mCherry is shown. Time = 0 min was set to the first frame after anaphase onset. See also Figure 3A.
(E) Immunofluorescence (IF) analysis and quantification of mitotic cells expressing ND cyclin B1 and transfected with either nontargeting control (NTC) small interfering RNA (siRNA) or siRNA duplexes targeting separase (n > 152 cells from three independent experiments). See also Figure S1G.
Scale bars represent 10 μm. See also Figure S1.