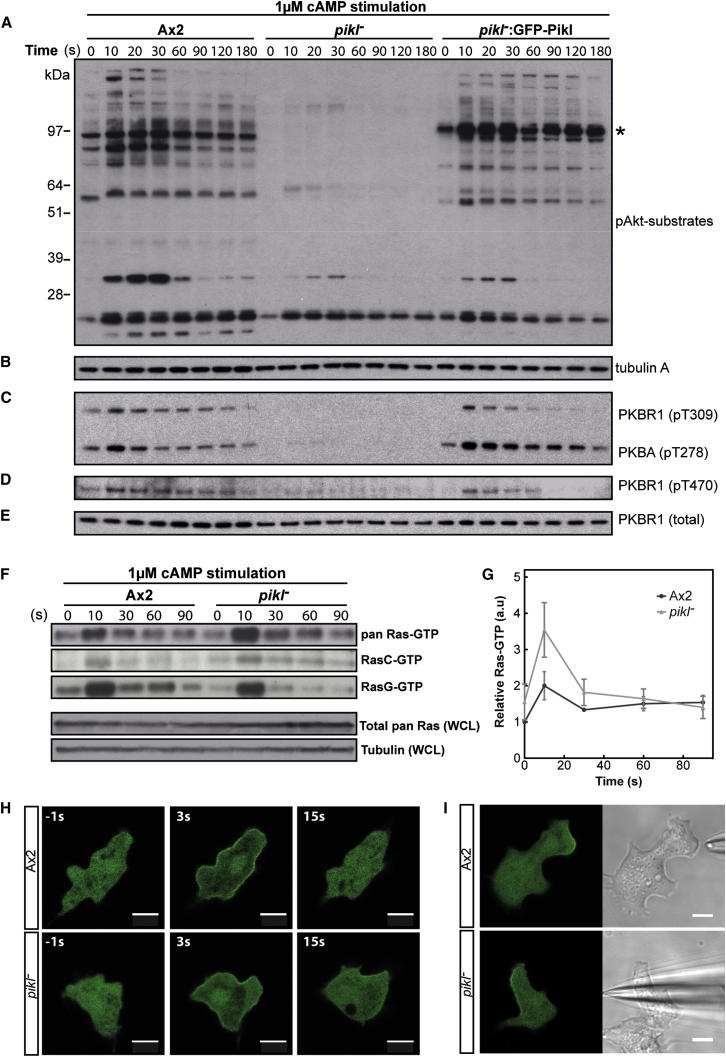
Figure 3.
Ras, but Not PKB/PKBR1, Is Efficiently Activated in Response to cAMP in pikI− Cells
(A–E) Representative western blots showing cell lysates from a time course after cAMP stimulation (at time zero). The primary antibodies listed below were used to blot.
(A) Anti-phospho-Akt-substrates (recognizing the phosphorylated substrates of PKBA and PKBR1). The asterisk shows the position of phosphorylated GFP-PikI; the band below 28 kDa is also seen in the pkbR1− strain [8] and is likely to be nonspecific.
(B) Anti-tubulin A (loading control).
(C) Anti-phospho-PKC-pan-zeta (recognizes the phosphorylated activation loop of PKBA and PKBR1).
(D) Anti-phospho-PDK1-docking motif (recognizes the phosphorylated hydrophobic motif of PKBR1).
(E) Anti-PKBR1 (total PKBR1 levels).
(F) Representative western blots showing Ras-GTP pulled down from lysates (400 μg protein per strain) of cells stimulated with cAMP over the time course indicated (n = 3). Blots were probed with anti-pan-Ras, anti-RasC, or anti-RasG as indicated. Two blots also show total Ras and tubulin in whole-cell lysate (WCL) as loading controls.
(G) Quantification of Ras-GTP pull-down; quantification shows mean (pan) Ras-GTP levels from anti-pan-Ras blots obtained in three independent experiments after normalization to Ax2 basal levels of Ras. Error bars represent ± SD (n = 3).
(H) Representative response of Raf1-RBD-GFP-expressing cells to uniform stimulation with cAMP.
(I) Example of localization of Raf1-RBD-GFP in cells responding to a steep cAMP gradient produced by a micropipette. Scale bars represent 5 μm.