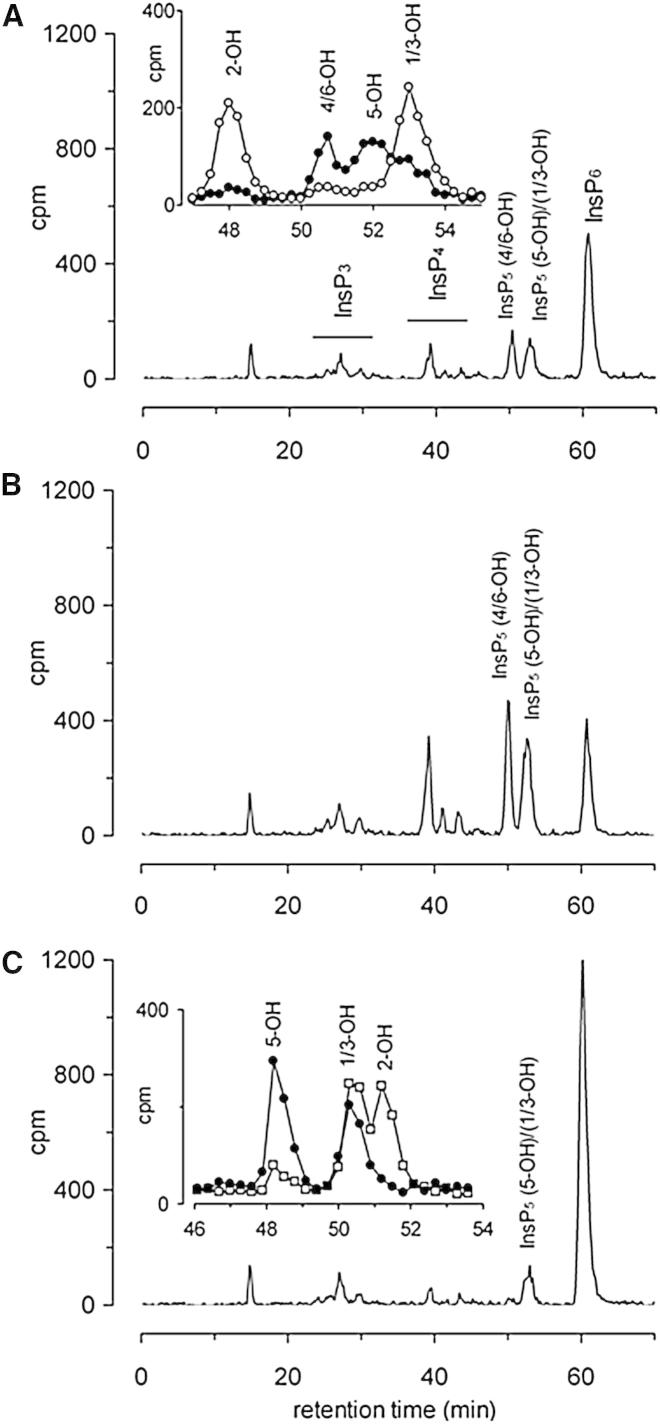
Figure 1.
Mutation of Active Site Residues Alters the Specificity of Initial Attack on InsP6 Substrate
(A–C) Products of reaction of native BtMinpp- (A), A31Y- (B), and R183D- (C) substituted enzyme with myo-inositol(1,[32P]2,3,4,5,6)P6 were resolved by Partisphere SAX HPLC. Mutated proteins were incubated at a concentration of 2.5 μg/ml and native protein at 0.25 μg/ml, with 1 mM InsP6. The regions of the chromatogram in which InsP3, InsP4, and specific InsP5 isomers elute are indicated in (A). For native enzyme (inset in A), reaction products were mixed with standards of myo-[2-3H]inositol (1,3,4,5,6)P5 (InsP5 [2-OH]), D/L- myo-[2-3H]inositol (1,2,4,5,6)P5 (InsP5 [1/3-OH]), and D/L- myo-[2-3H]inositol (1,2,3,4,5)P5 (InsP5 [4/6-OH]). Fractions, 0.25 min, were collected and radioactivity was estimated by scintillation counting; 3H, open circles; 32P, filled circles. For R183D-substituted enzyme (inset in C), the reaction products were additionally mixed with standards of myo-[14C]InsP5 [2-OH] and D/L- myo-[14C]InsP5 [1/3-OH] and resolved on a Adsorbosphere SAX HPLC column. This column separates InsP5 [2-OH] from InsP5 [1/3-OH]. Fractions, 0.25 min, were collected and radioactivity estimated by scintillation counting: 14C, open squares; 32P, filled circles.