Abstract
Aging is associated with a progressive decline in immune function (immunosenescence) resulting in an increased susceptibility to viral and bacterial infections. Here we show reduced expression of Toll-like receptor 1 (TLR1) in polymorphonuclear leukocytes (PMN) and an underlying age-dependent deficiency in PMN bioenergetics. In older (>65 years) adults, stimulation through TLR1 led to lower activation of integrins (CD11b and CD18), lower production of the chemokine IL-8, and lower levels of the phosphorylated signaling intermediate p38 MAP kinase than in PMN from younger donors (21-30 years). In addition, loss of CD62L, a marker of PMN activation, was reduced in PMN of older adults stimulated through multiple pathways. Rescue of PMN from apoptosis by stimulation with TLR1 was reduced in PMN from older adults. In seeking an explanation for effects of aging across multiple pathways, we examined PMN energy utilization and found that glucose uptake after stimulation through TLR1 was dramatically lower in PMN of older adults. Our results demonstrate a reduction in TLR1 expression and TLR1-mediated responses in PMN with aging, and reduced efficiency of bioenergetics in PMN. These changes likely contribute to reduced PMN efficiency in aging through multiple aspects of PMN function and suggest potential therapeutic opportunities.
Keywords: neutrophils, Toll-like receptors, p38 Map kinase signaling, aging, bioenergetics, integrins
INTRODUCTION
Aging is associated with a progressive decline in immune function -- termed immunosenescence -- that results in an increased susceptibility to viral and bacterial infections and decreased response to vaccines [1]. The adaptive immune system is heavily affected in aging, with a well-documented decline in humoral as well as cell-mediated immune responses [2]. Aging of the innate immune system is complex in spanning multiple cell types, activation states, and tissue context, and, while incompletely understood, it is characterized by dysregulation and inappropriate persistence of inflammatory responses [1]. Studies of the cellular mechanisms of aging broadly suggest roles for the accumulation of reactive oxygen species (ROS) leading to damage of biomolecules [3], an age-associated decrease of autophagy that reduces clearance of damaged mitochondria and cellular proteins [4], an NF-κB-dependent inflammatory state in the hypothalamus leading to immune-neuroendocrine decline [5], and dysregulation of glucose metabolism that may underlie many aspects of senescence and aging-related diseases [6]. Indeed, studies of caloric restriction in many species have been shown to extend lifespan [7].
Short-lived polymorphonuclear leukocytes (PMN), the most numerous cells in the human innate immune system, are newly released each day from bone marrow precursors and yet show effects of aging [8]. PMN are the first immune cells to migrate to pathogen-infected sites, and release potent reactive oxygen and nitrogen intermediates, along with granules containing abundant antimicrobial peptides, and more recently have been shown to play a role in cytokine production, extracellular trap formation, and regulation of adaptive immunity [8]. Deficiencies of PMN functions in aging include reduced recruitment, phagocytosis, release of cytokines and granules, and diminished microbial activity. These comprehensive deficiencies suggest an age-related dysfunction in signal transduction [9-12].
Antimicrobial host defense responses are triggered by Toll-like receptors (TLRs), pattern recognition receptors that recognize conserved molecular patterns on microbes [13, 14]. We have recently shown the effects of aging on TLR expression and function in innate immune cell types of a large cohort of younger and community-dwelling older adults. Age-associated deficits in monocytes include reduced surface expression of TLR1, reduced TLR1/2 function, reduced costimulatory responses associated with reduced vaccination efficiency [15, 16], and elevation in TLR5 that may contribute to the heightened ‘inflammaging” milieu [17]. Decreases in TLR expression and function in primary human dendritic cells were strongly associated with poor antibody response to influenza immunization [18] and reduced production of type I IFN to infection with West Nile virus [19], and macrophages from older adults showed dysregulation of TLR3 in response to infection with West Nile virus [20]. We have undertaken the present study to examine the age-related alterations of expression and function of TLRs on PMN, and investigate underlying mechanisms that may contribute to the impaired immunity observed in older adults.
RESULTS
Effect of aging on expression of TLRs in human PMN
To identify effects of aging on PMN, we recruited younger (N=38) and older (N=40) healthy individuals to compareTLR expression and function. Subjects were 57% female and 84% white (Table 1). PMN are short-lived cells that rapidly undergo apoptosis in vivo (8-12 hours), and in vitro may release highly active hydrolytic enzymes from their granules, which may degrade surface proteins [21]. In consideration of the special features of PMN, several measures were undertaken to maximize the reproducibility of our findings. Only samples that could be assessed within 2 hr of the blood collection were used, and to reduce the time to measurement, the expression of TLRs on PMN was assessed by flow cytometry using a whole blood assay. We found no significant difference in the percentage of PMN with age (data not shown), in keeping with previous reports [21].
Table 1.
Participant characteristics
| Young (N=38) | Old (N=40) | Total (N=78) | P-value* | |
|---|---|---|---|---|
| Age (y), mean(SD) (range) | 25.9(2.5) 22-30 | 74.5(7.0) 65-90 | 50.8(25.0) 22-90 | - |
| Female gender, N (%) | 21(55.3) | 24(60.0) | 45(57.7) | 0.82 |
| Race, N (%) | ||||
| White | 29(76.3) | 37(92.5) | 66(84.6) | 0.02 |
| Black | 1(2.6) | 2(5.0) | 3(3.9) | |
| Other | 8(21.1) | 1(2.5) | 9(11.5) | |
| Hispanic | 2(5.3) | 1(2.5) | 3(3.9) | 0.61 |
The p-values were calculated based on Fisher exact tests for categorical
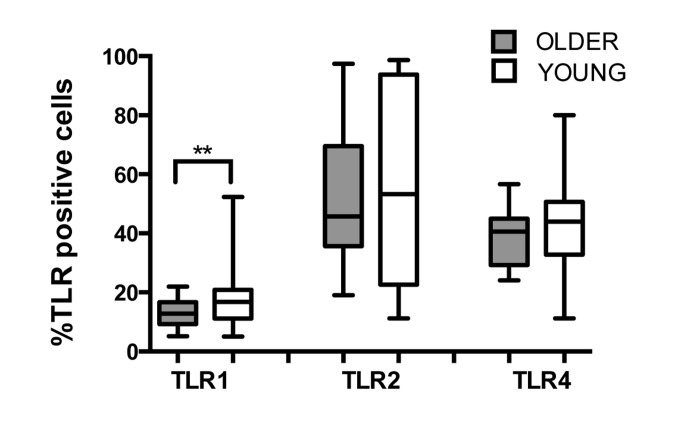
Human PMN express most TLRs (except TLR3) and respond to TLR ligand stimulation with production of pro-inflammatory cytokines and release of O2-, reflecting their active functions in the innate immune response [22]. We compared TLR expression between young and older adults and observed reduced expression of TLR1 by PMN of the older as compared to young adults (Fig. 1; p=0.02), which is consistent with previously described lower expression of TLR1 on monocytes and dendritic cells of older adults [15, 18]. Expression of TLR2 and TLR4 were equivalent in both age groups, as reported previously [23]. While TLR1 surface expression was reduced in PMN from older donors, no significant age-related differences were detected for mean fluorescent intensity of TLRs (data not shown), suggesting that the reduction in TLR1 surface expression may reflect age-related effects on intracellular trafficking pathways of TLR1, including changes in plasma membrane viscosity [24] or in PMN lipid raft domains in older individuals [25].
Figure 1. Effect of aging on expression of TLRs in human PMN.
Whole blood of younger (n=31) and older (n =22) adults was labeled for flow cytometry with lineage markers and TLRs at 4°C for 30 min following RBC lysis. Labeling was detected by LSR II. Data shown are % positive neutrophils for TLR surface expression. Values indicate the means ± SEM in young and older adults. Asterisks indicate statistical significance between younger and older cohort (Unadjusted t-test accounting for unequal variances, p < 0.02).
TLR1 stimulated activation of PMN and cytokine production is reduced in older adults
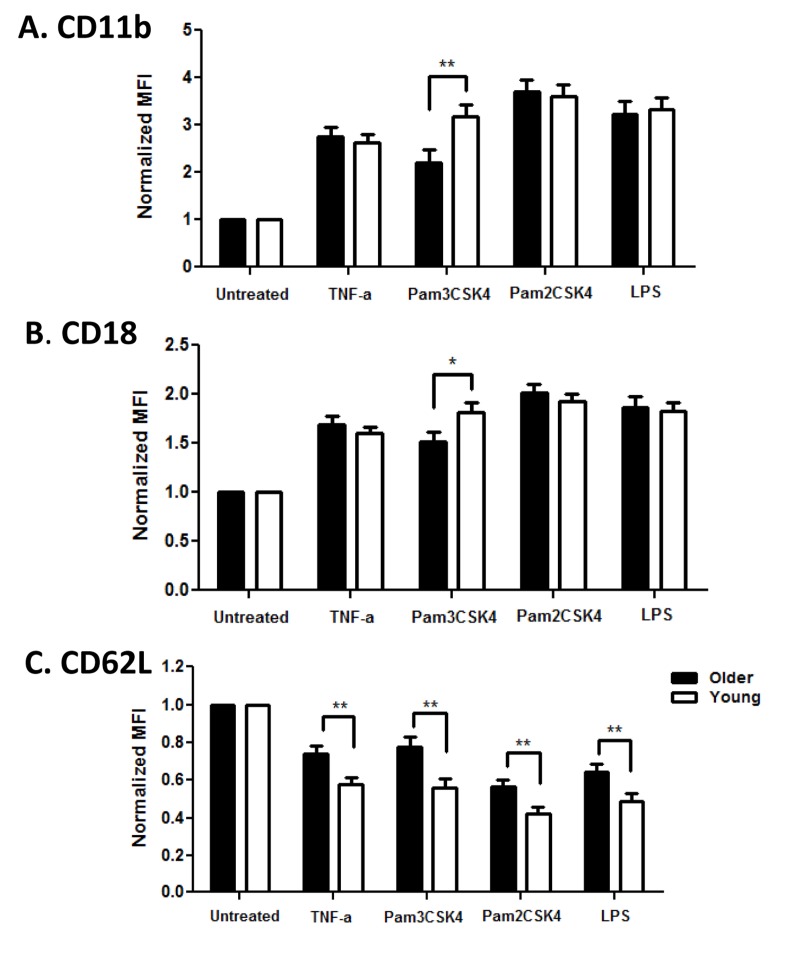
Activation of PMN leads to dramatic changes in adhesion to endothelial surfaces to promote diapedesis and PMN exit to the site of injury or infection, mediated in part by upregulation of integrins CD11b and CD18 and shedding of L-selectin (CD62L) from the cell surface [26]. We quantified expression of these adhesion markers to detect age-related differences in PMN functional efficiency. Baseline levels of CD11b and CD18 were not different between age groups, while CD62L was somewhat lower in the older cohort (data not shown). After stimulation with TLR ligands, PMN of both age groups upregulated CD11b and CD18, but the increase was significantly lower in PMN from older adults after stimulation through TLR1 (Pam3CSK4; Fig. 2A CD11b, p<0.01; Fig. 2B CD18, p<0.05). Notably, loss of CD62L expression after activation was also reduced on PMN from older adults, but this reduction was not limited to TLR1 stimulation (Fig 2C, p<0.01).
Figure 2. Age-associated alterations in PMN surface markers.
Whole blood of younger (n=32) and older (n =35) adults was stimulated with indicated ligands for 0, 15 min at 37 °C. Following RBC lysis, PMN were labeled for PMN markers at 4°C for 30 min. Flow cytometric labeling was detected by LSR II. Data shown are normalized MFI (Mean Fluorescence Intensity) of CD11b, CD18 and CD62L surface expression on PMN. Values indicate the means ± SEM in younger and older adults; asterisks indicate statistical significance between younger and older cohort (*P<0.05, **P < 0.01). For normalized CD11b and CD18 all stimulated PMN increased over time (P<0.001) and for CD62L all stimulated PMN decreased over time (P<0.001).
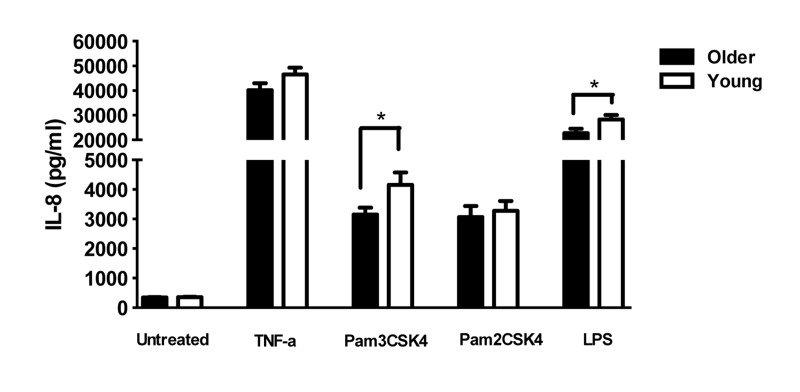
To determine the consequences of the lower levels of TLR1 on PMN in older adults, we quantified the production of cytokines from PMN of younger and older adults after stimulation by TLR ligands. PMN stimulated with ligands for TLR1 (Pam3CSK4) produced significantly higher IL-8 compared to PMN in medium alone, as expected [22],but levels from elderly adults were significantly lower than those from younger adults (Fig 3; p <0.05). Lower TLR1-induced cytokine production would be expected as a consequence of age-related reduced TLR1 expression. In contrast, stimulation with Pam2CSK4, the ligand for TLR2, elicited equivalent levels of IL-8 from PMN of both age groups (Fig. 3), likely reflecting the equivalent levels of TLR2 across age groups. Of note, production of IL-8 by PMN from older adults was also reduced after stimulation with LPS, the ligand for TLR4 (Fig. 3).
Figure 3. Effect of aging on TLR-stimulated production of cytokines by PMN.
In unstimulated vs TLR ligand-stimulated cells, the difference in the production of IL-8 in supernatants of PMN from younger (N=36) and older (N=34) adults. IL-8 was detected by ELISA after stimulation as shown. Values indicate the mean ± SEM concentration of IL-8 produced by PMN. Asterisks indicate statistical significance between younger and older cohorts (p < 0.05).
Mechanism of reduced signaling by PMN
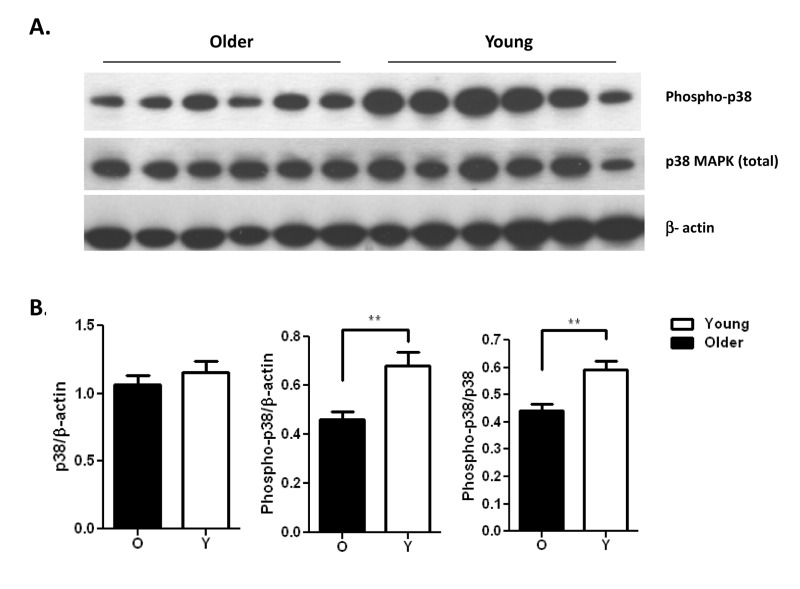
Reduced activation and production of cytokines by PMN in elderly adults may be significant for multiple PMN functions in vivo that depend on adhesion for recruitment and cytokines for activation of interacting immune cells [8]. To dissect the mechanism(s) underlying this reduction, we examined key components of the signaling cascade of PMN activation to assess age-related changes in signaling pathways. Signaling intermediates in activation of PMN include MAP kinase and NF-κB pathways [27, 28] and we have previously shown an age-dependent reduction in p38 MAPK pathway in TLR5 mediated production of IL-8 by monocytes [17]. Thus we assessed activation of p38 MAPK, signaling intermediate in PMN, and quantified PMN of both age groups for the level of phosphorylation of p38 MAP kinase after 15 min stimulation with the TLR1 ligand Pam3CSK4. While the total levels of p38 MAP kinase were not significantly different between age groups (Fig. 4A, middle row; ns), after stimulation the level of active phosphorylated p38 was significantly elevated in PMN of younger adults (Fig. 4A, top row). Quantitation of densitometry of blots from 21 pairs of younger and older subjects comparing the relative level of phosphorylated to total p38 shows significantly more activation for PMN of younger adults (Fig. 4B; p<0.01).
Figure 4. Pam3CSK4 stimulated neutrophils from the young show increased phosphorylation of p38 MAPK.
(A) immunoblot of total p38 MAPK and active phosphorylated p38 in a representative result of neutrophils from younger and older adults after stimulation with the TLR1/2 ligand Pam3CSK4 for 15 min. (B) Densitometry of immunoblot of p38 and phospho-p38 in neutrophils from 21 pairs of younger and older subjects after stimulation with Pam3CSK4 for 15min. Densitometry shows the means ± SEM of the ratio of total p38 and phospho-p38 to β-actin, and of phospho-p38 to total p38. Asterisks indicate statistical significance between younger and older cohort (** p < 0.01).
Reduced TLR1 mediated extension of PMN lifespan and cellular bioenergetics in older adults
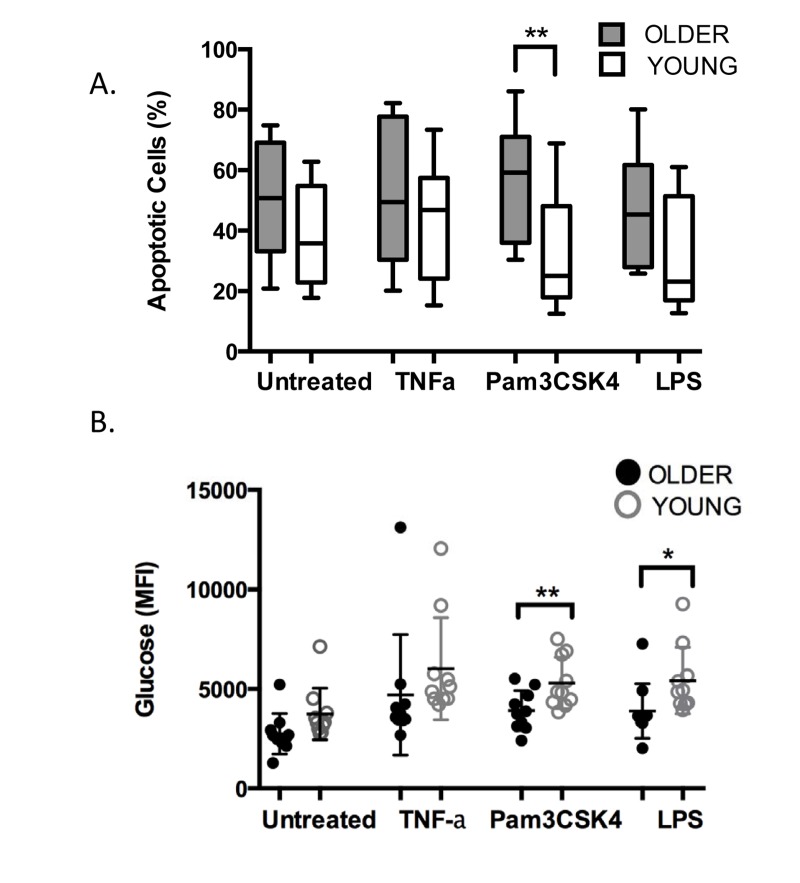
In response to immune activation by pathogens or by TLR stimulation, the PMN apoptosis program is delayed and the cells may persist at the site of infection for up to 6 hours [26, 29]. The age-related decrease we have noted in TLR1 expression and function may similarly result in a reduced ability to delay apoptosis in PMN of older adults. To assess this, we measured viability and the apoptosis marker Annexin V by flow cytometry in PMN from younger and older age groups after 18 hours in the absence or presence of stimulation (Fig. 5A). Without stimulation, PMN of both groups reached 38-50% positive for markers of apoptosis by 18 hr, with PMN from older donors showing higher levels of apoptotic cells as noted previously [30]. In response to stimulation, although the rate of apoptosis was reduced in PMN from both age groups, PMN from older adults still had higher rates of apoptosis than those of young adults. This age-related difference in rate of apoptosis reached significance when PMN were stimulated through TLR1(Fig. 5A, p< 0.04), reflecting the downstream effects of reduced expression of TLR1, and approached significance when stimulated through TLR4 (Fig. 5A, p=0.06).
Although short-lived, PMN are tremendously active cells, and with few mitochondria, they rely primarily on glycogen stores to support their activity [31]. At an inflammatory site, PMN have been shown to dramatically increase their content of glycogen by up to ten fold, presumably to support the high metabolic requirements of their immune-mediated program [31]. When we measured glucose accumulation in PMN using 2-NBDG, a fluorescent glucose marker, we noted somewhat lower levels of glucose in PMN of older adults at baseline that did not reach statistical significance (Fig 5B, ns). In response to stimulation, as expected, higher levels of glucose were detected in PMN of both age groups and accumulation of fluorescent glucose was higher across all stimulants as compared to untreated PMN (Fig. 5B, p<0.001). Notably, significantly lower levels of glucose accrued in PMN of older adults, particularly when stimulated through the TLR1 pathway (Fig. 5B, p< 0.02), and somewhat less through stimulation the TLR4 pathway (Fig. 5B, p<0.04).
Figure 5. Age-related alterations in TLR1-mediated stimulation of PMN.
PMN from younger and older adults (106/ml, n=10/group) were stimulated with TNF-α (20ng/ml), LPS (0.5μg/ml), or Pam3CSK4 (5μg/ml) in RPMI/10% human serum. Cells were incubated for 18 hr and labeled with Annexin V and PI for detection of apoptosis by flow cytometry (A). The level of fluorescent glucose (MFI of 2-NBDG) was quantified from aliquots of the same PMN by flow cytometry after 2 hr (B). Values indicate the mean ± SEM of apoptotic cells (A., ** P<0.04) and glucose level (B., * p< 0.04, ** p< 0.02) and asterisks indicate statistical significance between younger and older cohort.
DISCUSSION
Multiple impairments in innate and adaptive immune responses contribute to reduced immune responses in the elderly [1]. The aim of the present study was to investigate TLRs on PMN from older adults to determine expression levels and functional efficiency of receptors that are key to triggering antimicrobial host defense responses [13, 14]. We found that PMN from older donors express lower levels of TLR1 -- also noted on monocytes and dendritic cells [15, 18]-- which leads to reduced responses to TLR1 stimulation, reduced increases of adhesion molecules (integrins CD11b and CD18), and reduced production of the chemokine IL-8, mediated by reduced levels of activated p38 MAP kinase. Notably, these deficiencies are exacerbated in the frail elderly [32]. Although our study does not distinguish between whether the reduced expression of TLR1 alone accounts for the reduction in PMN function, or whether the remaining TLR1 may be impaired, lower efficiency of this critical pathway in aging is likely to play an important role in multiple PMN functions. In particular, recent reports have shown an association of reduced surface expression of TLR1 with increased susceptibility to M. tuberculosis [33].
Studies of the mechanisms of aging suggest that dysregulation of glucose metabolism may underlie many aspects of senescence and shortened lifespan [6, 7]. This is particularly relevant for PMN, which require robust energy homeostasis for the regulation of their function [34], with the decreased glucose response of PMN in aging resembling insulin resistance. Notably, decreased phagocytosis, killing of bacteria, and production of reactive oxygen species have long been observed in PMN from diabetic patients or in animal models of diabetes, which can be reversed by insulin treatment to restore glucose balance [35, 36]. Similarly, dysregulation of glucose has also been noted in PMN from patients with glycogen storage disease type 1b, whose neutrophils are reduced in number and function and show higher rates of apoptosis that were not responsive to G-CSF [37]. We have identified dramatic effects of aging on PMN accumulation of glucose stores, which are essential to fuel anti-microbial activity.
Evaluation of deficiencies in aging may in fact reveal adaptive strategies for successful survival. In our study, the older subjects (average age 74.5 years) remain healthy into old age, suggesting that the TLR1-mediated reduction in PMN function reported here, one of multiple deficiencies noted in immunosenescence [1], may be relevant to surviving other health complications, such as cancer or autoimmune diseases. Recent theories of aging and evidence that caloric restriction enhances longevity suggest paradoxically that reduction in anabolic processes may be beneficial to survival [38]. Caloric restriction is mediated in part by the TOR (target of rapamycin) pathway -- itself under the control of the circadian clock [39]— and while rapamycin inhibition of TOR signaling may be beneficial for longevity, during acute infection it can lead to inappropriate immune responses and increased tissue destruction [40]. Thus successful aging reflects a complex balance between lifespan extension and effective immune responses.
Nevertheless, the deficiency in bioenergetics in PMN described here may underlie several impairments noted in PMN function in aging and may also contribute to enhanced susceptibility to infections, pulmonary congestion, or recovery from burn injury noted in aging [9, 41, 42]. We and others have previously shown that PMN can act as a reservoir for bacterial [43, 44] or viral pathogens [45, 46], and PMN have recently been shown to transport antigen from the dermis to the bone marrow to activate CD8+ T cell responses [47]. It remains unknown whether aging may alter PMN function in elastase function related to colonization of intestinal microbiota or modulation of macrophage TLR4 expression [48, 49], or may contribute to significant PMN-related disease outcomes, including oxidase mediated regulation of chemokine receptors essential for recovery from intestinal inflammation [50]. As the effects of aging on PMN bioenergetics may be amplified in conditions that increase pathogen burdens and may contribute to deficits in the initiation of adaptive responses, our study suggests a potential therapeutic strategy to enhance innate immunity [6].
EXPERIMENTAL PROCEDURES
Study Subjects
Heparinized blood from healthy volunteers was obtained with written informed consent under a protocol approved by the Human Investigations Committee of Yale University School of Medicine. Study participants had no acute illness, and took no antibiotics or non-steroidal anti-inflammatory drugs within one month of enrollment. Demographic characteristics of subjects were collected at enrollment. Young adults (n=38) were aged 25.9 years (range 22-30), 55.3% female and 76.3% white. Older adults (n = 40) were aged 74.5 years (range 65-90), 60% female and 92.5% white (Table 1). Subjects were not statistically different for gender in this study. Self-reported information included demographic data, height, weight, medications, and comorbid conditions; immunocompromised individuals were excluded as described previously [17].
Cell stimulation and flow cytometry
For TLR detection, whole blood (180 μL/tube) was lysed by BD FACS Lysing Solution (BD Biosciences, CA) and PMN were stained with antibodies for lineage markers and TLRs as follows: APC-conjugated CD15, V500-conjugated CD45 (BD Biosciences, CA), PE-conjugated TLR 1, 4 and FITC-conjugated TLR2 (eBioscience, CA) for 30 min at 4°C. The immunostained cells were washed with wash solution and fixed with 1% paraformaldehyde. For stimulation assays, aliquots of whole blood were plated in 96-wells plate (180 μl/well) and incubated for 15 min in medium alone or with TNFα (20 ng/ml; R&D Systems, MN), or ligands for TLR 1 /2 (Pam3CSK4 5 μg/ml; Invivogen, CA), TLR2 (Pam2CSK4 1 μg/ml; Invivogen, CA), and TLR4 (LPS 0.5 μg/ml; Sigma, MO). Red blood cells were lysed and cell suspensions were labeled for 30 min at 4 °C with antibodies for PMN markers as follows: PE-CD11b, FITC-CD18, Pacific Blue-CD62L and V500-conjugated CD45 (BD Biosciences, CA) as described previously [32]. Data were acquired using an LSR II instrument (BD Biosciences, CA) and analyzed using FlowJo software (Tree Star, OR).
Isolation of human PMN and stimulation for cytokine ELISA, apoptosis, and glucose accumulation measurements
PMN were isolated from heparinized blood via dextran density sedimentation and hypotonic lysis of red blood cells as described [51]. Cells were routinely >95% pure and >99% viable. For apoptosis assays, PMN were stimulated for 18 hr in RPMI-1640 with 10% human serum and TNF-α (20ng/ml), LPS (0.5μg/ml), or Pam3CSK4 (5μg/ml) as indicated before labeling with Annexin V and propidium idodide and detection using LSRII. For quantitation of glucose accumulation, PMN were stimulated as above for 2 hours with 500 μM 2-NBDG (Sigma) added concurrently with stimulation; 2-NBDG levels in CD15+ cells were detected using LSRII. Supernatants from stimulated PMN incubations were stored frozen and cytokines were quantified by batch analysis enzyme-linked immunosorbent assays (ELISA) (OptEIA ELISA kit; BD Biosciences, CA).
Immunoblot analysis
PMNs (1 × 106/ well) were stimulated for 15 min with Pam3CSK4 (5 μg/ml). Cells were harvested using CelLytic M Cell Lysis buffer (Sigma, MO) containing protease inhibitor cocktail as described previously [17]. Immunoblots were probed with anti-phospho-p38 MAPK,anti-p38 MAPK-andanti-β-actin, developed using Amersham ECL Reagents (GE Healthcare), and scanned using Image J software.
Statistical analysis
Demographic characteristics were compared with Fisher exact tests for gender, race and ethnicity (Hispanic versus non-Hispanic), while age was sampled to differ, thus ranges are provided. The percentage of TLR positive PMN were compared between younger and older adults with t-test accounting for unequal variances. The Mean Fluorescence Intensity of normalized CD11b, CD18 and CD62L surface expression on PMN were analyzed with mixed effects models adjusting for sex and race, ligand, time, ligand by age group interaction, and ligand by time interaction as described [52]. Similar models were used for apoptosis and fluorescent glucose without the time elements. To address human heterogeneity, we used an unstructured covariance structure for each person to have a unique correlation among TLRs and times points. Statistical tests were 2-tailed, and p<0.05 considered significance. Multivariable analyses used SAS version 9.2 (SAS Institute, Cary, NC) and bivariate analyses used Prism 4.03 biostatistics package (Graphpad, CA).
Acknowledgments
Funding
This work was supported in part by the National Institutes of Health (HHS N272201100019C) and by the Yale Claude D. Pepper Older Americans Independence Center (P30 AG021342). The authors declare no competing financial interests. The authors are grateful to Donna Carrano, Barbara Siconolfi, Mark Trentalage, and Lin Zhang for valuable assistance, and the Yale IMAGIN team for insightful discussions.
FQ, XG, XW, and XY performed the experiments; RRM conceived the idea and drafted the manuscript; SC and HGA conducted statistical analysis; LKB and SEM evaluated and analyzed clinical data; all the authors reviewed the manuscript.
The authors of this manuscript have no conflict of interest to declare.
REFERENCES
- Shaw AC, Goldstein DG, Montgomery RR. Dysregulated innate immune function in aging. Nat Rev Immunol. 2013;13:875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd SD, Liu Y, Wang C, Martin V, Dunn-Walters DK. Human lymphocyte repertoires in ageing. Current opinion in immunology. 2013;25:511–515. doi: 10.1016/j.coi.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Wong E. Chaperone-mediated autophagy: roles in disease and aging. Cell research. 2014;24:92–104. doi: 10.1038/cr.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D. Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappaB and GnRH. Nature. 2013;497:211–216. doi: 10.1038/nature12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN. Metformin: do we finally have an anti-aging drug? Cell Cycle. 2013;12:3483–3489. doi: 10.4161/cc.26928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler SR. Caloric restriction: from soup to nuts. Ageing Res Rev. 2010;9:324–353. doi: 10.1016/j.arr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Jaillon S, Galdiero MR, Del Prete D, Cassatella MA, Garlanda C, Mantovani A. Neutrophils in innate and adaptive immunity. Seminars in immunopathology. 2013;35:377–394. doi: 10.1007/s00281-013-0374-8. [DOI] [PubMed] [Google Scholar]
- Stout-Delgado HW, Du W, Shirali AC, Booth CJ, Goldstein DR. Aging promotes neutrophil-induced mortality by augmenting IL-17 production during viral infection. Cell host & microbe. 2009;6:446–456. doi: 10.1016/j.chom.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels I, Jansen J, Rink L, Uciechowski P. Immunosenescence of polymorphonuclear neutrophils. The Scientific World Journal. 2010;10:145–160. doi: 10.1100/tsw.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T. Innate immunosenescence: Effect of aging on cells and receptors of the innate immune system in humans. Seminars in immunology. 2012;24:331–341. doi: 10.1016/j.smim.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Dalboni TM, Abe AE, de Oliveira CE, Lara VS, Campanelli AP, Gasparoto CT, Gasparoto TH. Activation profile of CXCL8-stimulated neutrophils and aging. Cytokine. 2013;61:716–719. doi: 10.1016/j.cyto.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Antiviral signaling through pattern recognition receptors. Journal of biochemistry. 2007;141:137–145. doi: 10.1093/jb/mvm032. [DOI] [PubMed] [Google Scholar]
- van Duin D, Thomas V, Mohanty S, Montgomery RR, Ginter S, Fikrig E, Allore HG, Medzhitov R, Shaw AC. Age-associated Defect in Human TLR1 Function and Expression. J Immunol. 2007;178:970–975. doi: 10.4049/jimmunol.178.2.970. [DOI] [PubMed] [Google Scholar]
- van Duin D, Allore HG, Mohanty S, Ginter S, Newman FK, Belshe RB, Medzhitov R, Shaw AC. Prevaccine Determination of the Expression of Costimulatory B7 Molecules in Activated Monocytes Predicts Influenza Vaccine Responses in Young and Older Adults. J Infect Dis. 2007;195:1590–1597. doi: 10.1086/516788. [DOI] [PubMed] [Google Scholar]
- Qian F, Wang X, Zhang L, Chen S, Piecychna M, Allore H, Bockenstedt LK, Malawista SE, Bucala R, Shaw A, Fikrig E, Montgomery RR. Age-associated elevation in TLR5 leads to increased inflammatory responses in the elderly. Aging Cell. 2012;11:104–110. doi: 10.1111/j.1474-9726.2011.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda A, Qian F, Mohanty S, van Duin D, Newman FK, Zhang L, Chen S, Towle V, Belshe RB, Fikrig E, Allore HG, Montgomery RR, Shaw AC. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol. 2010;184:2518–2527. doi: 10.4049/jimmunol.0901022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian F, Wang X, Zhang l, Lin A, Zhao H, Fikrig E, Montgomery RR. Impaired interferon signaling in dendritic cells from older donors infected in vitro with West Nile virus. J Infect Dis. 2011;203:1415–1424. doi: 10.1093/infdis/jir048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong K-F, Delroux K, Wang X, Qian F, Arjona A, Malawista SE, Fikrig E, Montgomery RR. Dysregulation of TLR3 impairs the innate immune response to West Nile virus in the elderly. J Virol. 2008;82:7613–7623. doi: 10.1128/JVI.00618-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord JM, Butcher S, Killampali V, Lascelles D, Salmon M. Neutrophil ageing and immunesenescence. Mech Ageing Dev. 2001;122:1521–1535. doi: 10.1016/s0047-6374(01)00285-8. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–2669. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- Fulop T, Larbi A, Douziech N, Fortin C, Guerard KP, Lesur O, Khalil A, Dupuis G. Signal transduction and functional changes in neutrophils with aging. Aging Cell. 2004;3:217–226. doi: 10.1111/j.1474-9728.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- Perskin MH, Cronstein BN. Age-related changes in neutrophil structure and function. Mech Ageing Dev. 1992;64:303–313. doi: 10.1016/0047-6374(92)90086-s. [DOI] [PubMed] [Google Scholar]
- Fortin CF, Larbi A, Lesur O, Douziech N, Fulop T., Jr Impairment of SHP-1 down-regulation in the lipid rafts of human neutrophils under GM-CSF stimulation contributes to their age-related, altered functions. J Leukoc Biol. 2006;79:1061–1072. doi: 10.1189/jlb.0805481. [DOI] [PubMed] [Google Scholar]
- Sabroe I, Dower SK, Whyte MK. The role of Toll-like receptors in the regulation of neutrophil migration, activation, and apoptosis. Clin Infect Dis. 2005;41(Suppl 7):S421–426. doi: 10.1086/431992. [DOI] [PubMed] [Google Scholar]
- Cloutier A, Ear T, Blais-Charron E, Dubois CM, McDonald PP. Differential involvement of NF-kappaB and MAP kinase pathways in the generation of inflammatory cytokines by human neutrophils. J Leukoc Biol. 2007;81:567–577. doi: 10.1189/jlb.0806536. [DOI] [PubMed] [Google Scholar]
- Kim CH, Lee KH, Lee CT, Kim YW, Han SK, Shim YS, Yoo CG. Aggregation of beta2 integrins activates human neutrophils through the IkappaB/NF-kappaB pathway. J Leukoc Biol. 2004;75:286–292. doi: 10.1189/jlb.0103038. [DOI] [PubMed] [Google Scholar]
- Francois S, El Benna J, Dang PM, Pedruzzi E, Gougerot-Pocidalo MA, Elbim C. Inhibition of neutrophil apoptosis by TLR agonists in whole blood: involvement of the phosphoinositide 3-kinase/Akt and NF-kappaB signaling pathways, leading to increased levels of Mcl-1, A1, and phosphorylated Bad. J Immunol. 2005;174:3633–3642. doi: 10.4049/jimmunol.174.6.3633. [DOI] [PubMed] [Google Scholar]
- Fortin CF, Lesur O, Fulop T., Jr Effects of TREM-1 activation in human neutrophils: activation of signaling pathways, recruitment into lipid rafts and association with TLR4. Int Immunol. 2007;19:41–50. doi: 10.1093/intimm/dxl119. [DOI] [PubMed] [Google Scholar]
- Robinson JM, Karnovsky ML, Karnovsky MJ. Glycogen accumulation in polymorphonuclear leukocytes, and other intracellular alterations that occur during inflammation. The Journal of cell biology. 1982;95:933–942. doi: 10.1083/jcb.95.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juthani-Mehta M, Guo X, Shaw AC, Towle V, Ning Y, Wang X, Allore HG, Fikrig E, Montgomery RR. Innate Immune Responses in the Neutrophils of Community Dwelling and Nursing Home Elders. J Aging Sci. 2014;2:115. doi: 10.4172/2329-8847.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uciechowski P, Imhoff H, Lange C, Meyer CG, Browne EN, Kirsten DK, Schroder AK, Schaaf B, Al-Lahham A, Reinert RR, Reiling N, Haase H, Hatzmann A, et al. Susceptibility to tuberculosis is associated with TLR1 polymorphisms resulting in a lack of TLR1 cell surface expression. J Leukoc Biol. 2011;90:377–388. doi: 10.1189/jlb.0409233. [DOI] [PubMed] [Google Scholar]
- Chou JY, Jun HS, Mansfield BC. Neutropenia in type Ib glycogen storage disease. Current opinion in hematology. 2010;17:36–42. doi: 10.1097/MOH.0b013e328331df85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba-Loureiro TC, Munhoz CD, Martins JO, Cerchiaro GA, Scavone C, Curi R, Sannomiya P. Neutrophil function and metabolism in individuals with diabetes mellitus. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica et al. 2007;40:1037–1044. doi: 10.1590/s0100-879x2006005000143. [DOI] [PubMed] [Google Scholar]
- Sunahara KK, Sannomiya P, Martins JO. Briefs on insulin and innate immune response. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2012;29:1–8. doi: 10.1159/000337579. [DOI] [PubMed] [Google Scholar]
- Kuijpers TW, Maianski NA, Tool AT, Smit GP, Rake JP, Roos D, Visser G. Apoptotic neutrophils in the circulation of patients with glycogen storage disease type 1b (GSD1b) Blood. 2003;101:5021–5024. doi: 10.1182/blood-2002-10-3128. [DOI] [PubMed] [Google Scholar]
- Donehower LA. Rapamycin as longevity enhancer and cancer preventative agent in the context of p53 deficiency. Aging. 2012;4:660–661. doi: 10.18632/aging.100494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khapre RV, Kondratova AA, Patel S, Dubrovsky Y, Wrobel M, Antoch MP, Kondratov RV. BMAL1-dependent regulation of the mTOR signaling pathway delays aging. Aging. 2014;6:48–57. doi: 10.18632/aging.100633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldenauer ME, McClellan SA, Berger EA, Hazlett LD. Mammalian target of rapamycin regulates IL-10 and resistance to Pseudomonas aeruginosa corneal infection. J Immunol. 2013;190:5649–5658. doi: 10.4049/jimmunol.1203094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez CR, Hirano S, Cutro BT, Birjandi S, Baila H, Nomellini V, Kovacs EJ. Advanced age exacerbates the pulmonary inflammatory response after lipopolysaccharide exposure. Critical care medicine. 2007;35:246–251. doi: 10.1097/01.CCM.0000251639.05135.E0. [DOI] [PubMed] [Google Scholar]
- Nomellini V, Brubaker AL, Mahbub S, Palmer JL, Gomez CR, Kovacs EJ. Dysregulation of neutrophil CXCR2 and pulmonary endothelial icam-1 promotes age-related pulmonary inflammation. Aging and disease. 2012;3:234–247. [PMC free article] [PubMed] [Google Scholar]
- Gresham HD, Lowrance JH, Caver TE, Wilson BS, Cheung AL, Lindberg FP. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J Immunol. 2000;164:3713–3722. doi: 10.4049/jimmunol.164.7.3713. [DOI] [PubMed] [Google Scholar]
- Laskay T, van Zandbergen G, Solbach W. Neutrophil granulocytes as host cells and transport vehicles for intracellular pathogens: apoptosis as infection-promoting factor. Immunobiology. 2008;213:183–191. doi: 10.1016/j.imbio.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Lu M, Lau LT, Lu J, Gao Z, Liu J, Yu AC, Cao Q, Ye J, McNutt MA, Gu J. Neutrophils may be a vehicle for viral replication and dissemination in human H5N1 avian influenza. Clin Infect Dis. 2008;47:1575–1578. doi: 10.1086/593196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F, Kong K-F, Dai J, Qian F, Zhang L, Brown CR, Fikrig E, Montgomery RR. A paradoxical role for neutrophils in the pathogenesis of West Nile virus. J Infect Dis. 2010;202:1804–1812. doi: 10.1086/657416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy D, Perrin H, Abadie V, Benhabiles N, Boissonnas A, Liard C, Descours B, Reboulleau D, Bonduelle O, Verrier B, Van Rooijen N, Combadiere C, Combadiere B. Neutrophils transport antigen from the dermis to the bone marrow, initiating a source of memory CD8+ T cells. Immunity. 2012;37:917–929. doi: 10.1016/j.immuni.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Gomes FL, Moniz-de-Souza MC, Alexandre-Moreira MS, Dias WB, Lopes MF, Nunes MP, Lungarella G, DosReis GA. Neutrophils activate macrophages for intracellular killing of Leishmania major through recruitment of TLR4 by neutrophil elastase. J Immunol. 2007;179:3988–3994. doi: 10.4049/jimmunol.179.6.3988. [DOI] [PubMed] [Google Scholar]
- Gill N, Ferreira RB, Antunes LC, Willing BP, Sekirov I, Al-Zahrani F, Hartmann M, Finlay BB. Neutrophil elastase alters the murine gut microbiota resulting in enhanced Salmonella colonization. PLoS ONE. 2012;7:e49646. doi: 10.1371/journal.pone.0049646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KL, Goel G, Sokol H, Manocha M, Mizoguchi E, Terhorst C, Bhan AK, Gardet A, Xavier RJ. p40phox expression regulates neutrophil recruitment and function during the resolution phase of intestinal inflammation. J Immunol. 2012;189:3631–3640. doi: 10.4049/jimmunol.1103746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Booth CJ, Paley MA, Wang X, DePonte K, Fikrig E, Narasimhan S, Montgomery RR. Inhibition of neutrophil function by two tick salivary proteins. Infect Immun. 2009;77:2320–2329. doi: 10.1128/IAI.01507-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda A, Chen S, Shaw AC, Allore HG. Statistical approaches for analyzing immunologic data of repeated observations: A practical guide. Journal of immunological methods. 2013;398-399:19–26. doi: 10.1016/j.jim.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]