Figure 3. The sequences of the membrane-buried regions of selected cyclotides and their lipophilicity profiles.
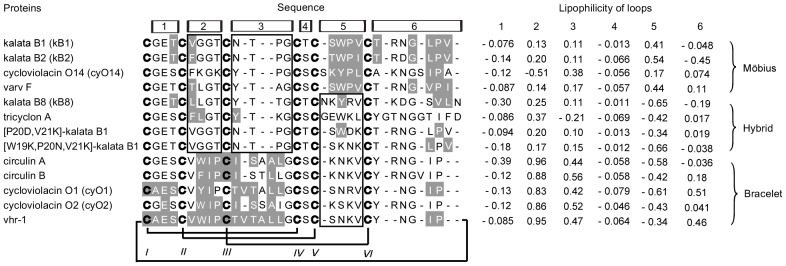
The multiple sequence alignment shows all of the cyclotides for which 3D structures (based on solution-phase NMR studies) are available. These sequences were used as template candidates for homology modeling of the cyclotides of unknown structure. The cysteine of loop 1 was arbitrarily selected as the N-terminus for the multiple sequence alignments. The residues required for cyclization, i.e. the conserved C-terminal residue (Asn or Asp) and N-terminal residue (Gly), are positioned within loop 6. The membrane-buried residues are highlighted with a gray background. OPM was used to predict the orientation of the selected cyclotides on the membrane. Among the members of the Möbius subfamily, many of the loop 5 and 6 residues are membrane-buried. Among the bracelet cyclotides, large proportions of loops 2 and 3 are buried. Only a couple of residues from the cyclotides of the hybrid subfamily penetrate the membrane interface. The lipophilicity of the loops was calculated as the total lipophilicity (LS) of all of the residues within the loop. It should be noted that the membrane-buried regions identified by OPM are consistent with those exhibiting high lipophilicity.
