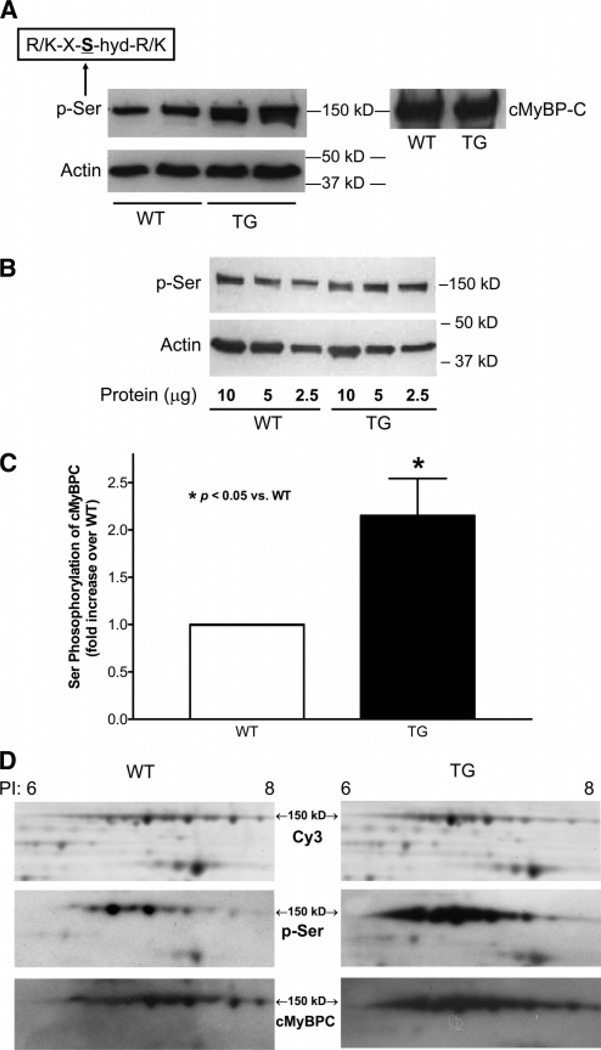
Figure 1.
Western blotting on the ventricular myofibrillar proteins extracted from 12-month-old WT or TG mouse hearts for Ser phosphorylation. (A) Representative result of SDS–PAGE gel electrophoresis and Western blotting using p-SerPKC antibody. Ten micrograms of sample was loaded in each lane. Membrane was stripped and then reprobed for actin (42 kDa) as control for loading. Cardiac MyBPC was identified at 150 kDa by Western blotting using anti-cMyBPC antibody. (B) Western blotting on serially diluted sample proteins using p-SerPKC antibody. The result confirmed the finding in panel A. (C) Quantitative densitometry analysis on the results from the Western blotting using p-SerPKC antibody; n = 5 (from five pairs of hearts). The density of the p-Ser of cMyBPC was normalized to that of the stripped and reprobed actin in the same sample. The data were then presented as fold increase in Ser phosphorylation of cMyBPC over that of the WT hearts. The paired, one-tailed t-test was used for statistical analysis. p < 0.05, statistically significant. (D) 2D gel analysis on the Ser phosphorylation of cMyBPC. Equal amounts of the samples from WT or TG hearts (50 µg from each) were labeled with Cy3 and underwent 2D gel electrophoresis on separate gels as shown in the first row. The proteins were transferred simultaneously to the same PVDF membrane, and Western blotting was performed first using p-SerPKC antibody (second row). Cardiac MyBPC was detected at 150 kDa. The membrane was then stripped and reblotted for cMyBPC (third row). n = 2.