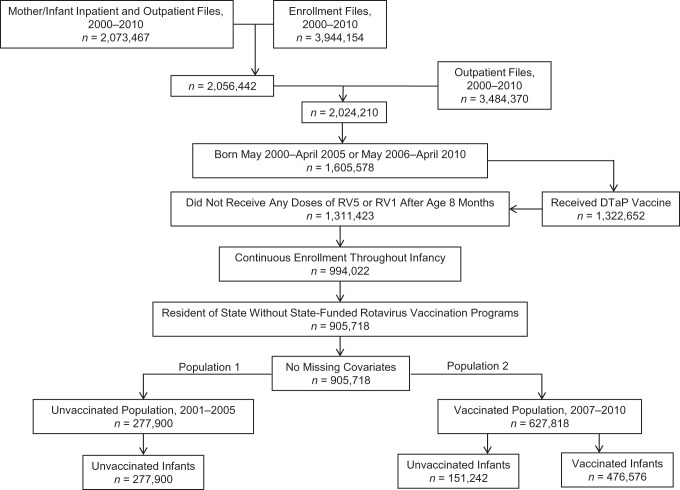
Figure 2.
Derivation of the unvaccinated population (population 1) and the vaccinated population (population 2) in the rotavirus vaccine effectiveness cohort study of US commercially insured infants and children 8–20 months of age, 2001–2005 and 2007–2010. Births were identified using International Classification of Diseases, Ninth Revision, Clinical Modification, codes in inpatient and outpatient records. These records were restricted to infants 0 years of age and to females 10–50 years of age. Twenty-three of 905,718 infants were excluded from this cohort in the final rotavirus gastroenteritis analysis because their cohort entry date (8-month birthday) equaled their cohort exit date (rotavirus gastroenteritis hospitalization date (n = 4) or loss of health plan enrollment date (n = 19)); for the same reasons, 40 infants were excluded from the final acute gastroenteritis analysis. DTaP, diphtheria, tetanus, and acellular pertussis vaccine; RV1, monovalent rotavirus vaccine; RV5, pentavalent rotavirus vaccine.