Fig. 1.
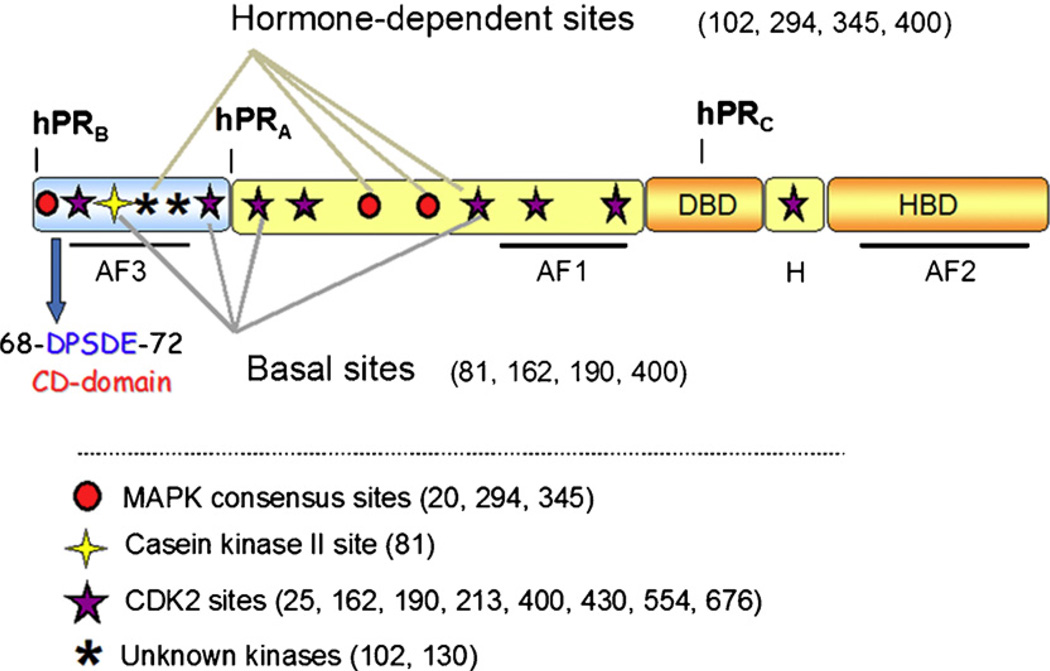
Schematic of progesterone receptor. All three PR isoforms (PR-A, PR-B and PR-C) are transcribed from the same gene, containing distal and proximal promoters, and created via differential use of two internal translational start sites. Shown are three transcription activation function (AF) domains, the B-upstream segment (BUS), the DNA-binding domain (DBD), the hinge region (H) and the hormone-binding domain (HBD). PR is phosphorylated basally, as well as in response to hormone. Shown here are the various sites of phosphorylation as determined in vitro and in vivo, and kinases that are likely responsible for phosphorylation at these sites. The putative common docking (CD) domain is located within the BUS, a segment unique to PR-B. This is the proposed site for MEK1 binding to PR.