Abstract
The receptor tyrosine kinase (RTK) Met is known to be over-expressed in canine osteosarcoma (OSA). In human cancers, the RTKs Met, epidermal growth factor receptor (EGFR) and Ron are frequently co-expressed and engage in heterodimerization, altering signal transduction and promoting resistance to targeted therapeutics. We found that EGFR and Ron are expressed in canine OSA cell lines and primary tissues, EGFR and Ron are frequently phosphorylated in OSA tumour samples, and Met is co-associated with EGFR and Ron in canine OSA cell lines. Transforming growth factor alpha (TGFα) and hepatocyte growth factor (HGF) stimulation induced amplification of ERK1/2 and STAT3 phosphorylation in OSA cells and Met was phosphorylated following TGFα stimulation providing evidence for receptor cross-talk. Lastly, treatment of OSA cells with combined gefitinib and crizotinib inhibited cell proliferation in an additive manner. Together, these data support the notion that Met, EGFR and Ron interact in OSA cells and as such, may represent viable targets for therapeutic intervention.
Keywords: cross-talk, EGFR, Met, osteosarcoma, Ron
Introduction
Osteosarcoma (OSA) is the most common primary bone tumour in dogs. Following standard treatment consisting of amputation and adjuvant chemotherapy, approximately 60% of affected dogs die within 1 year following diagnosis, with less than 10–20% living beyond 2 years.1 No significant improvements in outcome have occurred in the past 15 years, and the development of new therapeutic strategies will likely depend on more detailed characterization of the molecular abnormalities that underlie this disease.
Aberrant receptor tyrosine kinase (RTK) function often plays an important role in the development and progression of many cancers.2 The basic mechanism for RTK activation involves growth factor (ligand)-induced homodimerization followed by transphosphorylation of key tyrosine residues within the catalytic domain,3 leading to phosphorylation of additional sites important for adaptor protein docking and signal transduction.4 However, many RTKs have now been shown to associate with other related or unrelated RTKs and these interactions result in functional downstream signalling through both receptors. A classic example of this is the ErbB family consisting of four RTKs that frequently form heterodimers with one another. Heterodimerization occurs among members of the insulin and platelet-derived growth factor (PDGF) receptor families as well.5–7 In addition, some RTKs associate with integrins, plexins and other cell surface receptors and signal through these when stimulated with ligand.
We and others have previously demonstrated that the RTK Met is aberrantly expressed and functional in canine OSA cell lines.8–10 Met, along with the RTK Recepteur d'origine nantais (Ron), comprise the HGF/Met receptor family, and the tyrosine kinase domains of these receptors share 80% identity.11,12 Met interacts with other receptors including integrins, class B plexins, CD44, G protein-coupled receptors and other RTKs including epidermal growth factor receptor (EGFR). Furthermore, Ron has been shown to contribute to Met-associated biological responses (reviewed in 13).
Recent studies suggest Met and EGFR, or Met and Ron, engage in cross-talk that alters signal transduction in certain cancers. For example, kinase inactive mutant receptors were used to show that ligand-induced activation of Met results in transphosphorylation of Ron, and vice versa.14 In human ovarian carcinoma cell lines which co-expressed Met and Ron, simultaneous addition of the ligand for Ron, macrophage stimulating protein, and HGF, the ligand for Met, enhanced ovarian cancer cell invasiveness.15 Met and epidermal growth factor (EGF) family members are often co-expressed in carcinomas. Examples include Her2/neu/Met in breast cancer and gastric carcinoma in humans.16,17 Co-immunoprecipitation of a Met–EGFR complex was identified in human epidermal carcinoma and lung cancer cells.18,19 In human non-small cell lung carcinoma (NSCLC) cell lines, it was determined that amplified Met drives the activity of EGFR family members and that mutated and amplified EGFR can drive Met activity.19,20 EGFR-dependent Met phosphorylation is seen following ligand stimulation of lung or carcinoma cells with EGF or transforming growth factor alpha (TGFα). Finally, HGF stimulation was shown to promote transactivation of EGFR in multiple cell lines, including human retinal pigment epithelial cells, NSCLC and mouse mammary carcinoma.21–23
Interactions between Met/Ron and Met/EGFR may elicit co-ordinated cellular responses that confer some biologic advantage to tumour cells. In comparison to Met, Ron is less efficient as a kinase, thus Met/Ron complexes may result in more efficient Ron transphosphorylation by Met, thereby enhancing the duration/strength of signal than could be induced by a Ron/Ron homodimer.14 Ron, EGFR and Met activation leads to signalling through the phosphatidyl inositol-3 kinase (PI3K) and Ras/mitogen-activated (Ras/Raf/MAPK) pathways, thus cross-talk between these RTKs may promote signal amplification, subsequently enhancing cell growth, survival and motility.24–27
Few studies have investigated the role of EGFR in OSA. EGFR expression was identified in 55% of human OSA tumour samples by tissue microarray and correlated with ERK expression.28,29 EGFR expression and genomic gains at the EGFR locus are prevalent in human OSA tumours, which also commonly harbour deletions at the phosphatase and tensin homolog (PTEN) locus.30 Interestingly, expression and amplification of EGFR (80 and 23%, respectively) were frequently observed in high-grade OSAs and were associated with improved prognosis.31 Lower EGFR expression in resection specimens following chemotherapy versus initial biopsies suggested that the decreased EGFR expression contributed to chemotherapy resistance.31 Even less is known regarding the role of Ron in OSA. Recently, Ron expression was found in a broad panel of childhood sarcomas including primary OSAs.32 In this study, siRNA mediated down regulation of Ron expression restored sensitivity to the insulin-like growth factor 1 receptor (IGF1R) kinase inhibitor BMS-536924 in sarcoma cell lines highly resistant to BMS-536924.32 Together, these findings suggest that both Ron and EGFR may be dysregulated in OSA.
The purpose of this study was to interrogate canine OSA for expression of Ron and EGFR, to assess the functional interactions of Met, Ron and EGFR in OSA cell lines, and to evaluate the effects of inhibition of Met and EGFR in OSA cell lines.
Materials and methods
Cell lines and reagents
Canine OSA cell lines D17, OSA8, OSA16 and OSA36 were provided by Dr Jaime Modiano (AMC Cancer Research Institute, Denver, CO, USA). Canine primary OSA tumour samples were obtained from The Ohio State University, College of Veterinary Medicine Biospecimen Repository (OSU, Columbus, OH, USA). Cells were maintained in RPMI-1640 medium supplemented with 10% foetal bovine serum, nonessential amino acids, sodium pyruvate, HEPES, penicillin, streptomycin and L-glutamine. Recombinant human HGF (rhHGF, Biosource, Camarill, CA, USA) was used to stimulate canine and human Met. Recombinant human tumour growth factor alpha (rhTGFα, Abcam, Cambridge, MA, USA) was used to stimulate canine and human EGFR. Immunoprecipitation and immunoblotting of Met and EGFR was performed using anti-Met polyclonal antibody (Millipore, Temecula, CA, USA), anti-Ronβ polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-EGFR polyclonal antibody (Millipore). Protein loading was assessed using β-actin (Santa Cruz Biotechnology). Phosphorylated Met was detected using pTyr1230/1234/1235 (Biosource) and pTyr1234/1235 (Cell Signaling Technology, Danvers, MA, USA). Phosphorylated EGFR was detected using pTyr1068 (Cell Signaling Technology). Additional antibodies utilized included β-actin (Santa Cruz Biotechnology), pSer473-AKT, and total AKT, phospho-ERK1/2, and total ERK1/2, pTyr705-STAT3 and total STAT3 (Cell Signaling Technology). Gefitinib was purchased from Selleck Chemicals (Houston, TX, USA). Crizotinib was a gift from Pfizer (Groton, CT, USA).
Phospho-RTK array profiling
To assess relative phosphorylation of 42 different RTKs in primary OSA tumour biopsies, a Proteome Profiler™ Human Phospho-RTK Array Kit was used (R&D Systems, Minneapolis, MN, USA). Briefly, frozen tumour samples were pulverized using a frozen mortar and pestle. The resulting powder was resuspended in liquid nitrogen, and transferred to a 1.5 mL microcentrifuge tube. Once the liquid nitrogen evaporated away, the samples were allowed to thaw on ice and resuspended in tissue lysis buffer containing 20 mM Tris-HCl, 2 mM EDTA, 137 mM NaCl, 10 μg mL–1 aprotinin, 10 μg mL–1 leupeptin, 1 mM sodium orthovanadate, 1% IGEPAL® CA 630 and 10% glycerol. Samples were rocked for 1 h at 4 °C, centrifuged for 15 min at 14 000 × g at 4 °C, and supernatants were collected. Bradford protein quantification assay was performed on the extracts using BioRad Reagent (BioRad, Hercules, CA, USA) with 100 μg of protein lysate used for each RTK array following the manufacture's instructions.
RT-PCR
For reverse transcriptase-PCR to detect message for Ron and EGFR, total RNA was extracted from primary OSA tumour samples or 15 × 106 OSA cells using TRIzol® reagent (Invitrogen, Carlsbad, CA, USA), and cDNA was synthesized. Custom oligonucleotides were synthesized including primer pairs designed for detection of canine (c) Ron and EGFR (Table 2). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers were used as an internal control (Eurofins MWG Operon, Huntsville, AL, USA).
Table 2.
Primers for Ron and EGFR reverse transcriptase-PCR
| Primer | Sequence 5′–3′ |
|---|---|
| cRon2606F | GGTCGTTTGGTGTGTTGCTATGG |
| cRon2875R | CATAGGCTGCCGGCAGCTGCACAT |
| CEGFR086F | GCCCAAGATCCCATCCATTGC |
| CEGFR350R | CTTGTACACTGTGCCGAATGC |
| hGAPDH520F | ACCACAGTCCATGCCATCAC |
| hGAPDH971R | TCCACCACCCTGTTGCTGTA |
Immunohistochemistry for Ron and Met
Immunohistochemistry was performed on a canine primary OSA tumour tissue microarray containing 124 tumour samples (courtesy of C. Khanna, Comparative Oncology Program, National Cancer Institute). The samples included in the array were from dogs participating in several studies. There were 105 samples with sufficient clinical information to be included in the disease free interval and survival analysis. Within the OncoLAR (octreotride pamoate long-acting release analogue of somatostatin) study, treatment groups included dogs treated with carboplatin plus OncoLAR (n = 22) or carboplatin plus placebo (n = 21).33 Another study included dogs treated with STEALTH liposome-encapsulated cisplatin (SPI-77, n = 14) or carboplatin (n = 12).34 OPLA-Pt (open cell polylactic acid containing cisplatin biodegradable implant) study groups included an OPLA-Pt plus carboplatin (n = 12), and OPLA-Pt plus carboplatin and adriamycin (n = 8). Finally, dogs were treated with carboplatin alone (n = 15) or alternating carboplatin and adriamycin (n = 1). Disease free intervals and overall survival times were similar among all these treatment groups.
Every 50th section of the tumour tissue microarray is stained with H&E and reviewed. The cellularity of the cores is variable, from 100 to ~10%; however, unlike other tissue microarrays the balance of the core is rarely normal bone, but most commonly osteoid. Deparaffinization with xylene baths followed by rehydration with graded alcohols was performed. Samples were pretreated with antigen Dako antigen retrieval solution (Carpinteria, CA, USA) followed by endogenous peroxidase quenching, blocking with non-fat dry milk, and incubation with anti-Ron and anti-Met antibody, Dako streptavidin, and diaminobenzodine (DAB)-chromogen solution. Haematoxylin counterstaining, dehydration and coverslip mounting was then performed. Antibody staining was visually semi-quantitatively scored with 0, 1, 2 and 3 corresponding to no, low, intermediate or high expression, respectively. The number of samples within each of the semi-quantitative scores (0 to 3) was 43, 26, 26 and 16 for Met expression, and 66, 16, 13 and 8 for Ron expression. Clinical and histopathologic case history and outcome associations linked with the tumour samples were used to correlate expression with disease free interval and survival time represented in Kaplan–Meier curves.
Co-immunoprecipitation, immunoprecipitation and western blotting
OSA cells were collected, washed once with phosphate buffered saline (PBS) and resuspended in lysis buffer consisting of 20 mM Tris-HCl pH 8.0, 137 mM NaCl, 10% glycerol, 1% IPEGAL CA-630, 10 mM ethylenediaminetetraacetic acid (EDTA), 1 mg mL–1 aprotinin, 1 mg mL–1 leupeptin, 1 mg mL–1 pepstatin A, 1 mM phenylmethylsulphonyl fluoride, 1 mM sodium orthovanadate and 10 mM sodium fluoride (all from Sigma, St. Louis, MO, USA) for 1 h at 4 °C. Protein was quantified by the Bradford Assay for each of the sample lysates and an equivalent amount of protein (2 mg) was used for co-immunoprecipitation and (50 μg) simultaneously for western blotting. The co-immunoprecipitation supernatant was precleared by incubation with anti-rabbit IgG beads (eBiosciences, San Diego, CA, USA) for 30 min at 4 °C followed by immunoprecipitation using anti-Met (Millipore), anti-EGFR (Millipore, Temecula, CA, USA), anti-Ronβ antibody (Santa Cruz Biotechnology, Inc.) and the Rabbit Trueblot Kit (eBiosciences). For inhibitor plus growth factor treatments, 5 × 106 OSA8 cells were serum starved in C0 medium for 2 h followed by inhibitor treatment with 2 μM gefitinib, 2 μM crizotinib or a combination of both for 2 h in C0. The cells were then stimulated 15 min with 100 ng mL–1 rhTGFα only, 50 ng mL–1 rhHGF only or 100 ng mL–1 rhTGFα + 50 ng mL–1 rhHGF. Met was immuno-precipitated from the lysates and 80 μg of protein derived from the cell lysates used for Met immuno-precipitation was used to detect phosphorylation and total protein levels of additional signalling intermediates via western blot analysis. Serum star-vationofOSA8cellsfor2 hfollowedby12,24or48 h incubation with rhTGFα (100 ng mL–1) was also performed.
Cell proliferation
To assess cell proliferation 2 × 105 OSA8 cells were seeded in a 96-well plate with quadruplicate wells treated with dimethyl sulfoxide (DMSO) as control or 5–50 μM gefitinib for 48 or 72 h to determine the 50% inhibitory concentration (IC50). Cell proliferation was then assessed following stimulation with rhHGF or rhTGFα, or a combination of these with gefitinib or crizotinib. Cells received media only as a control, crizotinib (2 μM) or gefitinib (10 μM) only or with rhHGF 50 ng mL–1, rhTGFα 100 ng mL–1 or a combination of these plus inhibitors for 72 h. Plates were harvested and analysed using the CyQUANT assay (Molecular Probes, Eugene, OR, USA). For all CyQUANT assays,fluorescencemeasurementsweremadeusing a plate reader (Molecular Devices, Sunnyvale, CA, USA) with excitation at 485 nm and emission detection at 530 nm. Relative cell number was calculated as a percentage of the control wells: absorbance of sample/absorbance of DMSO treated cells X 100.
Drug combination analysis
Experiments were performed in 96-well plates. OSA8 cells were seeded at a density of 1.5 × 105 cells per well in RPMI media containing 1% fetal calf serum (FCS). Stock solutions of gefitinib and crizotinib were prepared in DMSO. Serial dilutions (two-fold) for each compound were prepared, with the concentration range from .0625X to 4X the IC50 value of each drug. To assess potential synergistic interactions, the treatment regimen involved simultaneous treatment of cells with gefitinib and crizotinib for 72 h, in addition to controls consisting of cells treated with the individual compounds alone for 72 h. All treatments were performed in quadruplicate wells. Following drug treatment, the number of viable cells in each well was determined using CyQuant cell proliferation assay Kit. Drug interactions were analysed using CompuSyn 3.0.1 (ComboSyn, Paramus, NJ, USA), which is based on the median effect model of Chou and Talalay.35
Statistical analysis
Experiments were repeated three times. For assessment of the effect of growth factor with Met or EGFR inhibitor on cell proliferation, analysis of variance (ANOVA) models were used to analyse the data for each group separately. The multiple comparisons with a control were adjusted for using Dunnett's method.
Results
Receptor tyrosine kinase profiling in canine OSA
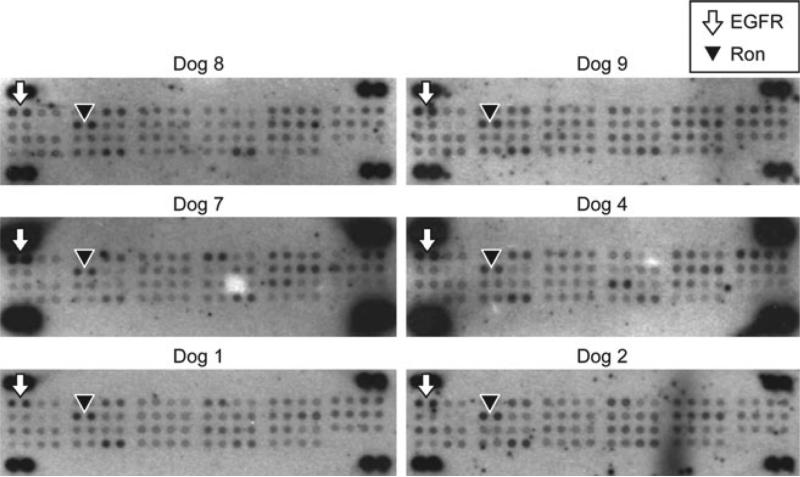
A phospho-receptor tyrosine kinase array (Proteome Profiler™ Human Phospho-RTK Array Kit, R&D Systems) was used to assess the activation of cell surface RTKs on primary OSA tumour samples. EGFR and Ron phosphorylation was consistently present in the primary OSA tissue specimens (Fig. 1). Of the primary tumours tested, 13/13 showed EGFR phosphorylation and 13/13 showed Ron phosphorylation. The results of the phospho-array profiling are summarized in Table 1.
Figure 1.
Phospho-RTK array profiling for canine primary OSA tumours. Frozen primary OSA tumour biopsies were used to determine phosphorylation of 42 different RTKs using the Proteome Profiler™ Human Phospho-RTK Array Kit. EGFR was consistently phosphorylated in canine OSA primary tumours. Ron was also consistently phosphorylated in canine OSA primary tumours. Table 1 summarizes the findings for the phospho-RTK array profiling.
Table 1.
Phospho-RTK profiling results for canine primary OSA tumours
| Axl | c-Ret | Dtk | EGFR | ErbB4 | EphB2 | EphA1 | FGFR3 | Insulin R | Ron | ROR1 | Tie-2 | VEGF R3 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Canine primary OSA tissue | Dog 1 | x | X | x | X | x | X | X | ||||||
| Dog 2 | x | x | X | X | x | x | x | X | x | |||||
| Dog 3 | x | X | X | x | X | X | X | |||||||
| Dog 4 | x | X | X | X | x | |||||||||
| Dog 5 | x | X | X | x | X | X | x | |||||||
| Dog 6 | x | x | X | X | X | x | x | X | X | x | x | |||
| Dog 7 | x | X | X | X | X | X | X | |||||||
| Dog 8 | x | X | X | X | X | x | X | x | ||||||
| Dog 9 | X | x | X | X | x | X | x | X | X | X | ||||
| Dog 10 | X | x | X | X | X | |||||||||
| Dog 11 | X | X | X | X | X | |||||||||
| Dog 12 | X | x | X | X | X | |||||||||
| Dog 13 | X | X | X | X | X |
EGFR and Ron are expressed in OSA primary tumours and cell lines
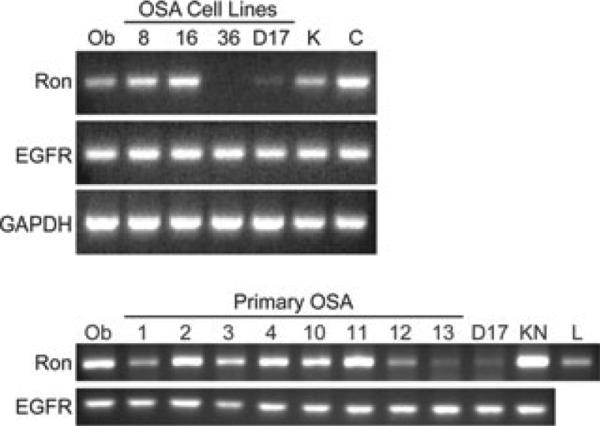
Total RNA was extracted from primary OSA tumour samples and OSA cells lines and cDNA was synthesized. Primer pairs designed for detection of canine Ron and EGFR are listed in Table 2. EGFR was consistently expressed in canine OSA cell lines (OSA8, OSA16 and D17) as well as primary OSA tumour tissue (Fig. 2A,B). Ron was expressed in the OSA8 and OSA16 and minimally in the D17 cells, but not OSA36 (Fig. 2A). Ron was consistently expressed in the primary OSA tumour tissues (Fig. 2B).
Figure 2.
Ron and EGFR are expressed in OSA cell lines and tumour specimens. Total RNA was extracted from primary OSA tumour samples or 15 × 106 cells using TRIzol® reagent followed by cDNA synthesis with reverse transcriptase-PCR to detect message for Ron and EGFR. EGFR was consistently expressed among all cell lines and primary tumour tissues evaluated. Ron was consistently expressed in primary OSA tumours and the OSA8 and OSA16 cell lines (numbers correspond to dogs listed in Table 1; Ob = K9 osteoblasts, KN = K9 TCC cell line, K = K9 kidney, C = K9 colon, L = K9 liver).
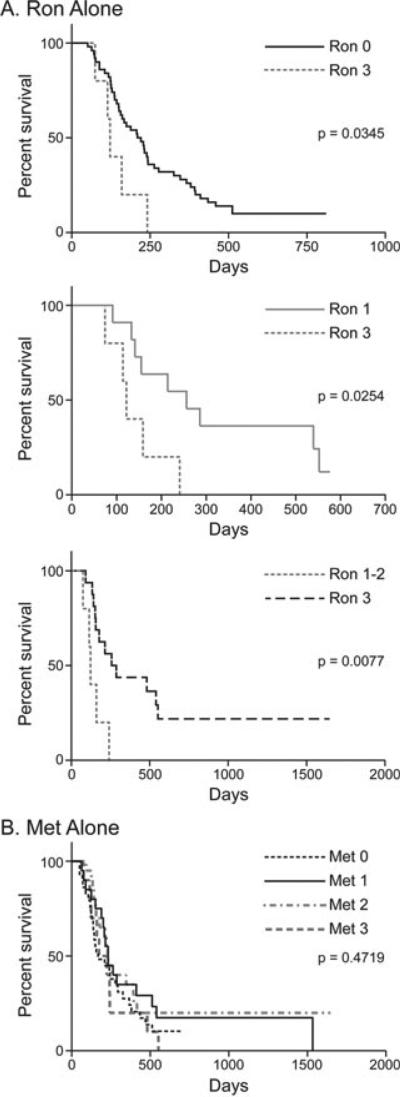
Ron expression is prognostic for survival in canine OSA
To determine the association between Ron and Met expression and disease free interval and survival, immunohistochemistry was performed using a canine OSA tissue microarray. Dogs with tumours that had high Ron expression experienced significantly decreased survival compared to those with intermediate (P = 0.0077), low (P = 0.0254) or no Ron expression (P = 0.0345) (Fig. 3A). Met expression alone was not associated with outcome in these patients (Fig. 3B). In addition, dogs with tumours that had a high Ron expression had a significantly decreased percent disease free interval compared to those with low Ron expression (P = 0.0158, data not shown). This indicates that Ron expression is significant in canine OSA.
Figure 3.
Ron expression is prognostic for survival in canine OSA. Kaplan–Meier survival curves were generated using a canine OSA primary tumour tissue microarray and data associated with patient outcome. (A) High Ron expression was associated with a significantly decreased survival compared to intermediate or low Ron expression. (B) Met alone was not associated with outcome in canine OSA.
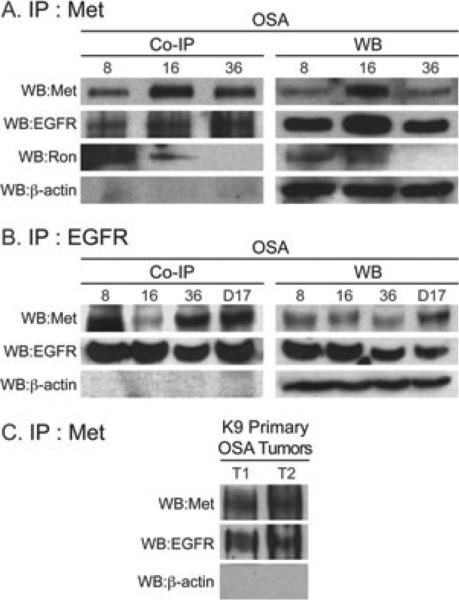
Met is associated with EGFR and Ron in canine OSA cell lines and primary OSA tumours
To determine if Met was associated with Ron or EGFR in canine OSA, co-immunoprecipitation experiments were performed in which Met was immunoprecipitated from canine OSA cell lines OSA8, OSA16 and OSA36 followed by western blotting to probe for Met, EGFR and Ron (Fig. 4A). The results show that Met is associated with EGFR in the OSA8, OSA16 and OSA36 cell lines and Ron in the OSA8 and OSA16 cell lines. The reciprocal experiment in which EGFR was immunoprecipitated followed by blotting for Met using OSA8, OSA16, OSA36 and D17 cell lines (Fig. 4B) confirmed that EGFR is associated with Met in these cell lines. In each experiment, a western blot was performed simultaneously to confirm expression of Met and Ron at the protein level (Fig. 4A,B, right side). In addition, Met was associated with EGFR in two canine primary OSA tumour tissue samples (Fig. 4C). The western blot results were concordant with the RT-PCR data (Fig. 2A) with no Ron protein expression and thus no association with Met seen in the OSA36 cell line (Fig. 4A). Met expression was variable, but this was expected as Met western blotting experiments usually require immunoprecipitation of the protein.
Figure 4.
Met, EGFR and Ron co-associate in canine OSA. Met, EGFR or Ron was immunoprecipitated from canine OSA cell lines followed by western blot analysis for Met, EGFR or Ron to determine interactions between these RTKs. (A and B) Met and EGFR interaction occurred in canine OSA cell lines and (C) primary canine OSA tumours. T1 = canine primary OSA tumour 1; T2 = canine primary OSA tumour 2 (A) Met was associated with Ron in canine OSA cell lines. Western blot analysis was performed simultaneously (A and B right side) to confirm protein expression of EGFR and Ron in the cell lines. [Note: immunoprecipitation (IP) is usually required for Met.]
TGFα stimulation potentially rescues canine OSA cells from Met inhibitor treatment
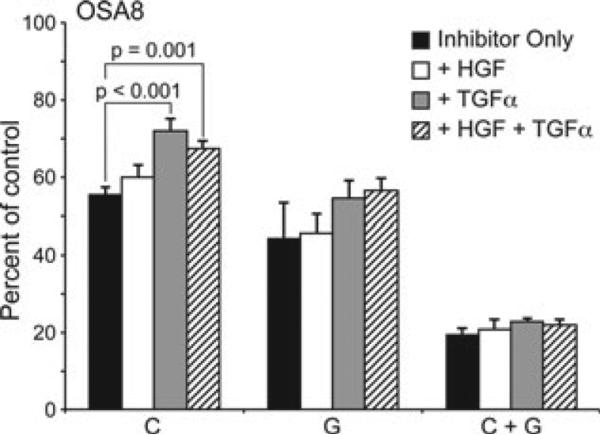
Crizotinib (Pfizer), is a specific Met small molecule inhibitor, although this drug also targets anaplastic lymphoma kinase. It is currently being evaluated in human phase I, II and III clinical trials.36 Gefitinib, a member of the 4-anilinoquinazoline class of compounds, is a specific and reversible inhibitor of EGFR. Its is currently approved to treat NSCLC patients refractory to chemotherapy.37–39 OSA8 cells stimulated with HGF, TGFα or both and treated with crizotinib (2 μM) or gefitinib (10 μM), or both, for 72 h showed a trend towards TGFα rescue of crizotinib-treated OSA8 cells (Fig. 5). This was significant for both the TGFα (P < 0.001) and TGFα plus HGF stimulated cells (P = .0001) that were treated with crizotinib, suggesting that EGFR may support the proliferation of OSA cells. Cell proliferation in response to ligand was modest for both HGF and TGFα. In addition, treatment with a combination of gefitinib and crizotinib enhanced the loss of cell proliferation in these cells compared to either inhibitor alone.
Figure 5.
TGFα stimulation potentially rescues canine OSA cells from Met inhibitor treatment. OSA8 cells were plated in C1 RPMI media. The cells were left unstimulated, or stimulated with HGF (50 ng mL–1), TGFα (100 ng mL–1) or both following treatment with crizotinib (C; 2 μM) or Gefitinib (G; 10 μM) or both for 72 h. TGFα stimulation of the cells promoted a significant rescue of crizotinib-treated OSA8 cells. In addition, treatment with a combination of gefitinib and crizotinib enhanced the loss of cell proliferation in these cells. The adjusted P-values following Dunnett's method and ANOVA analysis of the treatment combinations was P < 0.001 and P = 0.001 for the crizotinib plus TGFα and crizotinib plus TGF=α and HGF, respectively.
The combination of gefitinib and crizotinib results in additive growth inhibition
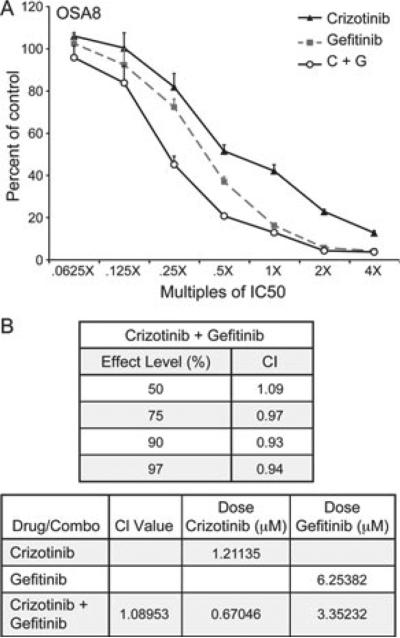
To evaluate if combinatorial inhibition of the Met and EGFR pathways promotes enhanced loss of cell proliferation, the OSA8 cell line was treated with gefitinib and crizotinib. Incubation with drug was performed using multiples of the IC50 ranging from 0.0625 to 4X. Gefitinib (0.625–40 μM) or crizotinib (0.25–8 μM) treatment reduced the proliferation of OSA8 cells in a dose dependent manner consistent with previous data (Fig. 6A). Treatment of OSA8 cells with both gefitinib and crizotinib resulted in an additive inhibition of cell growth, generating a combination index (CI) between 0.9 and 1.0 (Fig. 6B) at effect levels >50% ED50 (the point at which the combination inhibits cell growth by 50%).
Figure 6.
Gefitinib and crizotinib treatment promotes an additive effect for inhibiting canine OSA cell proliferation. OSA8 cells were plated in C1 RPMI media and treated for 72 h with gefitinib only, crizotinib only or a combination of gefitinib and crizotinib using concentrations ranging from 0.0625X to 4X their IC50 concentrations. (A) Cell proliferation was assessed using a CyQuant assay, which was then used for CompuSyn analysis. (B) The combination of gefitinib and crizotinib promoted an additive effect on inhibition of cell proliferation. The resulting CI values are shown for ED50 ≥ 50% (the point on the curve where the cell number is 50% of that in the untreated controls) (top table). The bottom table shows the data for ED50. To achieve 50% killing of the cells, it would require approximately twice the dose of gefitinib and crizotinib used individually compared to these drugs used in combination. The CI value definitions are as follows: 1.45–1.2 is moderately antagonistic, 1.2–1.1 is slightly antagonistic, 1.1–0.9 is additive, 0.9–0.85 is slightly synergistic, 0.85–0.7 is moderately synergistic and 0.7–0.3 is synergistic.
TGFα stimulation promotes Met phosphorylation at late but not early time points
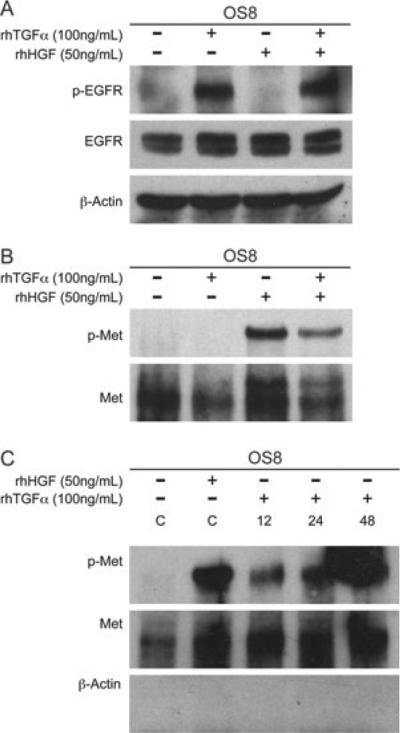
As OSA frequently expresses both EGFR and Met, we examined whether the ligand for EGFR or Met could activate the reciprocal receptor as a form of cross-talk given the association between these receptors demonstrated earlier. Stimulation of OSA8 cells with HGF or TGFα for 15 min did not result in phosphorylation of the reciprocal receptors (Fig. 7A,B). Recent data suggest that EGFR activation is capable of promoting Met phosphorylation in NSCLC cells, but that this phenomenon is observed at late (12–24 h) time points following stimulation. To determine whether cross-phosphorylation is similarly a late event in canine OSA, the OSA8 cells were serum starved and incubated with TGFα for 12, 24 or 24 h. In contrast to the short-term exposure, protracted incubation with TGFα promoted cross-phosphorylation of Met (Fig. 7C).
Figure 7.
Prolonged, not immediate TGFα stimulation promotes Met phosphorylation. OSA8 cells were serum starved for 2 h and then left unstimulated, or stimulated with HGF (50 ng mL–1), TGFα (100 ng mL–1) or a combination of both for 15 min. (A) Western blot analysis was performed on canine OSA8 cell lysates for EGFR phosphorylation. HGF stimulation did not promote EGFR phosphorylation. (B) Met was immunoprecipitated from canine OSA8 cells followed by western blot analysis. TGFα stimulation did not promote Met receptor phosphorylation. Thus, although a co-association was initially seen with Met and EGFR, a reciprocal activation of the receptors did not occur with an immediate HGF or TGFα stimulation. (C) For the prolonged TGFα stimulation, OSA8 cells were serum starved for 2 h before treatment with TGFα (100 ng mL–1) for a total of 12, 24 and 36 h followed by immunoprecipitation of Met. Western blot analysis was performed and showed phosphorylation of Met occurred with 12, 24 and 48 h of exposure to TGFα. Controls (C) included serum starved nonstimulated and 15-min HGF (50 ng mL–1) stimulated OSA8 cells. TGFα promoted Met phosphorylation that was evident at 12, 24 and 48 h.
Combined HGF and TGFα stimulation promotes enhanced ERK1/2 phosphorylation
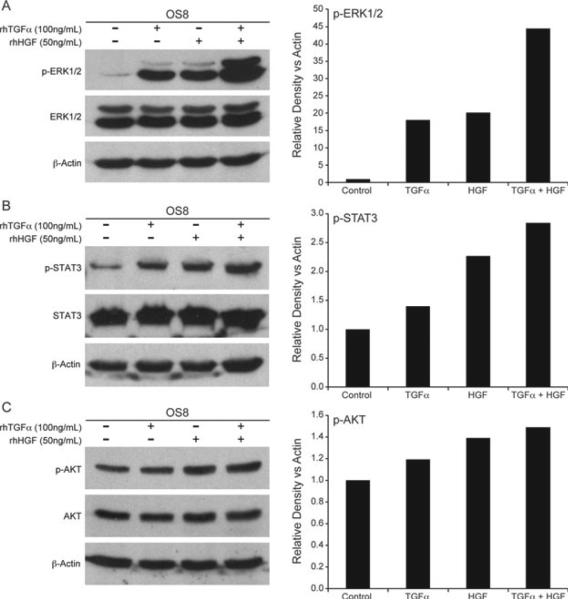
As the Met and EGFR pathways share similar downstream signalling pathways, we assessed ERK1/2, STAT3 and AKT phosphorylation following stimulation with HGF, TGFα or a combination of both. EGFR signalling in canine OSA is consistent with previously reported signalling pathways for EGFR including activation of ERK1/2 (Fig. 8A) and STAT3 (Fig. 8B) with TGFα stimulation. Furthermore, stimulation with HGF and TGFα did promote enhanced phosphorylation of signalling intermediates ERK1/2 and STAT3 (Fig. 8A,B), but not AKT (Fig. 8C). Interestingly, stimulation with both HGF and TGFα promoted an increase in ERK1 compared to the signal elicited by either growth factor alone (Fig. 8A).
Figure 8.
TGFα and HGF stimulation enhances ERK1/2 signalling in OSA8 cells. OSA8 cells were serum starved for 2 h and then left unstimulated, or stimulated with HGF (50 ng mL–1), TGFα (100 ng mL–1) for 15 min, or a combination of both. Western blot analysis was performed and showed (A) enhanced ERK1/2 phosphorylation, (B) mildly enhanced STAT3 phosphorylation, (C) and had no effect on AKT phosphorylation with combined HGF and TGFα stimulation compared to either alone. Western blot band density analysis was performed using ImageJ analysis software.
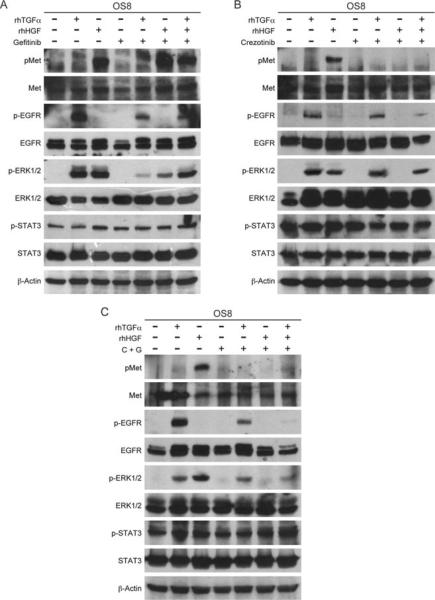
Met and EGFR inhibition confirms co-operative signalling in the MAPK pathway
To determine the effects of Met and EGFR inhibitor treatment on signalling induced by HGF, TGFα or a combination of both, OSA8 cells were serum starved for 2 h followed by treatment with crizotinib (2 μM), gefitinib (2 μM) or a combination of both for 2 h. Phosphorylation of STAT3 persisted despite treatment with gefitinib or crizotinib indicating that the EGFR and Met pathways are not the main drivers of STAT3 activation in these cells (Fig. 9A–C). ERK1/2 phosphorylation following HGF or TGFα stimulation was inhibited with the Met and EGFR inhibitors crizotinib (Fig. 9B) and gefitinib (Fig. 9C), respectively. No reciprocal inhibition of ERK1/2 phosphorylation was detected as crizotinib did not inhibit TGFα stimulated ERK1/2 phosphorylation (Fig. 9B), and gefitinib did not inhibit HGF stimulated ERK1/2 phosphorylation (Fig. 9A). Finally, ERK1/2 phosphorylation was maintained following HGF and TGFα stimulation in the presence of individual inhibitors (Fig. 9A,B) but was abrogated in the presence of both crizotinib and gefitinib (Fig. 9C).
Figure 9.
Met and EGFR inhibition confirms cooperative signalling in the MAPK pathway. OSA8 cells serum starved for 2 h and treated with (A) gefitinib (2 μM), (B) crizotinib (2 μM) or (C) both (C + G) for 2 h followed by stimulation for 15 min with HGF (50 ng mL–1), TGFα (100 ng mL–1), or both or left unstimulated. Immunoprecipitation for Met and western blot analysis for Met and signalling intermediates was performed.
Discussion
Despite treatment of OSA with limb amputation and chemotherapy, no significant improvement in survival times for dogs has been achieved in over a decade with less than 10–20% surviving greater than 2 years.40 An increased understanding of tumour biology has promoted selective targeting of cell signalling pathways as a mechanism for improving therapeutic efficacy. In particular, inhibition of RTKs is a strategy employed to target pathways that contribute to tumour cell proliferation, invasion and survival. Met, EGFR and Ron are RTKs expressed on a variety of cancer cell types and each has been found to have dysregulated expression in a variety of different cancers via over-expression and/or mutation. In addition, receptor cross-talk has been reported both between EGFR and Met in NSCLC and Ron and Met in cancers of the liver, ovary and bladder.15,41–43 Cross-talk between receptors is a mechanism by which growth factor receptors may affect the strength and duration of shared downstream signalling pathways. There are many mechanisms for crosstalk including transactivation of a receptor with a reciprocal ligand, transcriptional upregulation of the opposite receptor's ligand, lateral ligand independent activation, or direct binding of receptors providing stabilization and escape from endocytic degradation.13,22,41 Given the interaction of EGFR, Ron and Met in other cancer types, we wanted to examine the potential for interaction among these receptors in canine OSA.
Our data demonstrate that primary canine OSA exhibit expression of both Ron and EGFR, and that both receptors are phosphorylated in fresh tumour specimens based on RTK arrays. Met, EGFR and Ron have several major signalling pathways in common such as PI3K-AKT and ERK1/2-MAPK. This redundancy may provide a potential mechanism for compensatory signalling if inhibition of one of the RTK's occurs or possibly potentiation of signalling if multiple receptors are activated. In support of this idea, we demonstrated that Met, Ron and EGFR co-associated in canine OSA cell lines and tumour samples. As Ron is considered a less efficient kinase than Met, this association may promote a stronger or more sustained signal than Ron/Ron homodimers.14,44 Further-more, Ron-mediated activation of distal signalling intermediates following proximal inhibition of the IGF1R signalling axis in Ewings sarcoma cells has been documented32 supporting the notion that Ron activation influences other growth factor signalling pathways.
We next examined the expression level of Ron and its relationship to patient outcome and found that high Ron expression was associated with decreased survival and disease free interval in dogs with OSA. In human colorectal cancer, an aggressive subgroup of node negative breast cancer, and transitional cell carcinoma of the bladder, co-expression of Met and Ron was also associated with decreased survival.43,45,46 Together, these findings suggest that high Ron expression may promote heterodimer formation with Met (and possibly EGFR), thereby enhancing/modifying signalling through key cellular pathways that regulate oncogenic functions.
While the effects of Met/EGFR co-association have not previously been investigated in OSA, in human NSCLC, a synergistic effect of EGF and HGF on proliferation, downstream activation of signal transduction and additive effect on motility was demonstrated.47 It was also shown that constitutive phosphorylation of Met promoted resistance to EGFR kinase inhibitors in breast cancer and that decreasing Met kinase activity decreased EGFR tyrosine phosphorylation and proliferation in the presence of gefitinib.48 Furthermore, Met and EGFR co-immunoprecipitated in breast cancer cells in the presence and absence of gefitinib and down regulation of EGFR expression decreased Met constitutive phosphorylation.49 Thus, Met/EGFR interaction in OSA may provide an advantage to the tumour cells, permitting the cells to maintain biological functions in the face of limiting growth factor/ligand.
We did not observe evidence of EGFR/Met cross-talk when OSA cells were treated with TGFα for 15 min. However, a recent study with NSCLC cells expressing wild-type Met and EGFR showed delayed Met phosphorylation in response to EGF stimulation of EGFR beginning at 24 h and that Met signalling was required for maximal EGFR-induced invasion and motility.41 We therefore investigated whether delayed phosphorylation of Met may be occurring through TGFα stimulation of EGFR. As with the NSCLC cells, Met phosphorylation was present after 12, 24 and 48 h exposure of these OSA cells to TGFα. Cell proliferation in response to HGF and TGFα stimulation was not markedly increased; however, TGFα stimulated a mild rescue of cell proliferation in the OSA cells treated with the Met inhibitor, crizotinib after 72 h of culture. It is possible that the EGFR-Met cross-talk contributes to the TGFα-mediated rescue seen in the face of crizotinib treatment (Fig. 5). In NSCLC cells, the delayed Met phosphorylation was the result of ligand stimulated EGFR activation of c-Src followed by transcription of an unknown protein that enhanced Met phosphorylation. Delayed phosphorylation of Met may be a mechanism for EGFR to amplify the invasive phenotype as was the case in the NSCLC cells in which this phenomenon maximized cell motility and invasion. A similar effect may occur in OSA cells in which delayed Met phosphorylation may enhance previously initiated EGFR-mediated motility, invasion or proliferation without amplification or ligand stimulation of Met.
Combined treatment with gefitinib and crizotinib promoted an additive effect on inhibition of cell proliferation in the OSA cells. The combination studies demonstrate that the dose of each drug could be reduced by approximately 45% (if used in combination) to get 50% killing, relative to the doses needed to get the same level of cell kill when used as single agents. As stimulation with a combination of HGF and TGFα-enhanced ERK1/2 (particularly ERK1) and STAT3 phosphorylation, this additive effect may be due in part to dual inhibition of these pathways through Met and EGFR inhibition. The ERK1/2 pathway has multiple functions in a variety of cell types and appears to be important for antiapoptotic signals.50 For example, ERK induction can protect human ovarian carcinoma cells from chemotherapy-induced death.51 In one study, blocking ERK1/2 sensitized human malignant mesothlioma cells to doxorubicin through inhibition of expression of ATP-binding cassette genes.52 It is therefore possible that enhanced ERK1/2 signalling through co-ordinated activation of Met/EGFR/Ron signalling pathways helps protect OSA cells from chemotherapy-induced cell death.
A major problem associated with RTK monotherapy is the development of drug resistance. Previous reports have described acquired resistance to RTK inhibitors through multiple mechanisms. For instance, acquisition of additional point mutations in EGFR as well as c-Met amplification and subsequent HER3-dependent activation of PI3K in NSCLC are reported causes of resistance to gefitinib.53,54 In one study, combined inhibition of MET and EGFR kinases in MET-dependent NSCLC cells did not enhance their initial sensitivity to crizotinib, but did suppress the eventual emergence of drug-resistant clones after prolonged drug exposure.55 Activation of the EGFR pathway in these cells increased the number of crizotinib-resistant clones. Our data show that reduction of the dose of each drug by approximately 45% (if used in combination) achieves 50% killing, relative to the doses needed to get the same level of cell kill when used as single agents (Fig. 6B, bottom table). Thus, the relationship between Met and EGFR signalling indicates that combined use of Met and EGFR kinase inhibitors may be beneficial in OSA not only to enhance the effect of single agent therapy alone but also by reducing selection for drug-resistant clones.
In summary, our data demonstrate that Ron plays an important role in outcome for canine OSA patients. This may be related to direct interactions with Met as was documented for OSA cell lines and tumour samples, or may be associated with Ron activation of distal signalling targets that enhances the function/signalling of other kinases. Further studies are needed to identify the signalling pathways activated by Ron in OSA cells. We also demonstrated that Met and EGFR co-associate in OSA cells, inducing cross-talk through TGFα stimulation of Met as well as amplification of ERK1/2 phosphorylation following dual growth factor stimulation. Together, these data support the notion that Met, Ron and EGFR interact in canine OSA. This interaction proved functional for Met and EGFR, altering the manner in which these cells respond to growth factor stimulation. Future work evaluating the effects of inhibiting all three pathways in OSA cells in vitro and in vivo will help to define the therapeutic potential of these pathways.
Acknowledgements
The authors thank Mahmoud Abdel-Rasoul and Soledad Fernandez from the Center for Biostatistics, The Ohio State University for statistical analysis. We thank Mr Tim Vojt from The Ohio State University, Department of Veterinary Medicine, Biomedical Media Services, for his assistance. We would like to thank Pfizer for kindly providing crizotinib. Funds for this study was provided by the Morris Animal Foundation.
Footnotes
Conflict of interest
The authors have no conflicts of interest to declare.
References
- 1.Mueller F, Fuchs B, Kaser-Hotz B. Comparative biology of human and canine osteosarcoma. Anticancer Research. 2007;27:155–164. [PubMed] [Google Scholar]
- 2.Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. New England Journal of Medicine. 2005;353:172–187. doi: 10.1056/NEJMra044389. [DOI] [PubMed] [Google Scholar]
- 3.Weiss A, Schlessinger J. Switching signals on or off by receptor dimerization. Cell. 1998;94:277–280. doi: 10.1016/s0092-8674(00)81469-5. [DOI] [PubMed] [Google Scholar]
- 4.Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla Zonca P, Giordano S, Graziani A, Panayotou G, Comoglio PM. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77:261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 5.Wada T, Qian XL, Greene MI. Intermolecular association of the p185neu protein and EGF receptor modulates EGF receptor function. Cell. 1990;61:1339–1347. doi: 10.1016/0092-8674(90)90697-d. [DOI] [PubMed] [Google Scholar]
- 6.Tartare S, Ballotti R, Van Obberghen E. Interaction between heterologous receptor tyrosine kinases. Hormone-stimulated insulin receptors activate unoccupied IGF-I receptors. FEBS Letters. 1991;295:219–222. doi: 10.1016/0014-5793(91)81422-5. [DOI] [PubMed] [Google Scholar]
- 7.Kelly JD, Haldeman BA, Grant FJ, Murray MJ, Seifert RA, Bowen-Pope DF, Cooper JA, Kazlauskas A. Platelet-derived growth factor (PDGF) stimulates PDGF receptor subunit dimerization and intersubunit trans-phosphorylation. Journal of Biological Chemistry. 1991;266:8987–92. [PubMed] [Google Scholar]
- 8.Liao AT, McCleese J, Kamerling S, Christensen JG, London CA. A novel small molecule Met inhibitor, PF2362376, exhibits biological activity against canine osteosarcoma. Veterinary Comparative Oncology. 2007;5:177–196. doi: 10.1111/j.1476-5829.2007.00137.x. [DOI] [PubMed] [Google Scholar]
- 9.Fieten H, Spee B, Ijzer J, Kik MJ, Penning LC, Kirpensteijn J. Expression of hepatocyte growth factor and the proto-oncogenic receptor c-Met in canine osteosarcoma. Veterinary Pathology. 2009;46:869–877. doi: 10.1354/vp.08-VP-0155-F-FL. [DOI] [PubMed] [Google Scholar]
- 10.Raffaella De M, Silvia M, Selina I, Martina O, Emanuela M, Andrea B, James GC, Bartolomeo B, Roy AL, Paolo B, Maria Flavia Di R. Met oncogene activation qualifies spontaneous canine osteosarcoma as a suitable pre-clinical model of human osteosarcoma. The Journal of Pathology. 2009;218:399–408. doi: 10.1002/path.2549. [DOI] [PubMed] [Google Scholar]
- 11.Wagh PK, Peace BE, Waltz SE. Met-related receptor tyrosine kinase Ron in tumor growth and metastasis. Advances Cancer Research. 2008;100:1–33. doi: 10.1016/S0065-230X(08)00001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comoglio PM, Boccaccio C. The HGF receptor family: unconventional signal transducers for invasive cell growth. Genes Cells. 1996;1:347–354. doi: 10.1046/j.1365-2443.1996.37037.x. [DOI] [PubMed] [Google Scholar]
- 13.Lai AZ, Abella JV, Park M. Crosstalk in Met receptor oncogenesis. Trends in Cell Biology. 2009;19:542–551. doi: 10.1016/j.tcb.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Follenzi A, Bakovic S, Gual P, Stella MC, Longati P, Comoglio PM. Cross-talk between the proto-oncogenes Met and Ron. Oncogene. 2000;19:3041–3049. doi: 10.1038/sj.onc.1203620. [DOI] [PubMed] [Google Scholar]
- 15.Maggiora P, Lorenzato A, Fracchioli S, Costa B, Castagnaro M, Arisio R, Katsaros D, Massobrio M, Comoglio PM, Flavia Di Renzo M. The RON and MET oncogenes are co-expressed in human ovarian carcinomas and cooperate in activating invasiveness. Experimental Cell Research. 2003;288:382–389. doi: 10.1016/s0014-4827(03)00250-7. [DOI] [PubMed] [Google Scholar]
- 16.Shattuck DL, Miller JK, Carraway KL, III, Sweeney C. Met receptor contributes to trastuzumab resistance of Her2-overexpressing breast cancer cells. Cancer Research. 2008;68:1471–1477. doi: 10.1158/0008-5472.CAN-07-5962. [DOI] [PubMed] [Google Scholar]
- 17.Nakajima M, Sawada H, Yamada Y, Watanabe A, Tatsumi M, Yamashita J, Matsuda M, Sakaguchi T, Hirao T, Nakano H. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer. 1999;85:1894–1902. doi: 10.1002/(sici)1097-0142(19990501)85:9<1894::aid-cncr3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 18.Jo M, Stolz DB, Esplen JE, Dorko K, Michalopoulos GK, Strom SC. Cross-talk between epidermal growth factor receptor and c-Met signal pathways in transformed cells. The Journal of Biological Chemistry. 2000;275:8806–8811. doi: 10.1074/jbc.275.12.8806. [DOI] [PubMed] [Google Scholar]
- 19.Guo A, Villen J, Kornhauser J, Lee KA, Stokes MP, Rikova K, Possemato A, Nardone J, Innocenti G, Wetzel R, Wang Y, MacNeill J, Mitchell J, Gygi SP, Rush J, Polakiewicz RD, Comb MJ. Signaling networks assembled by oncogenic EGFR and c-Met. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:692–697. doi: 10.1073/pnas.0707270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reznik TE, Sang Y, Ma Y, Abounader R, Rosen EM, Xia S, Laterra J. Transcription-dependent epidermal growth factor receptor activation by hepatocyte growth factor. Molecular Cancer Research. 2008;6:139–150. doi: 10.1158/1541-7786.MCR-07-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Z, Du R, Jiang S, Wu C, Barkauskas DS, Richey J, Molter J, Lam M, Flask C, Gerson S, Dowlati A, Liu L, Lee Z, Halmos B, Wang Y, Kern JA, Ma PC. Dual MET-EGFR combinatorial inhibition against T790M-EGFR-mediated erlotinib-resistant lung cancer. British Journal of Cancer. 2008;99:911–922. doi: 10.1038/sj.bjc.6604559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu KP, Yu FS. Cross talk between c-Met and epidermal growth factor receptor during retinal pigment epithelial wound healing. Investigative Ophthalmology and Visual Science. 2007;48:2242–2248. doi: 10.1167/iovs.06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonine-Summers AR, Aakre ME, Brown KA, Arteaga CL, Pietenpol JA, Moses HL, Cheng N. Epidermal growth factor receptor plays a significant role in hepatocyte growth factor mediated biological responses in mammary epithelial cells. Cancer Biology and Therapy. 2007;6:561–570. doi: 10.4161/cbt.6.4.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clinical Cancer Research. 2001;7:2958–2970. [PubMed] [Google Scholar]
- 25.Pedersen MW, Meltorn M, Damstrup L, Poulsen HS. The type III epidermal growth factor receptor mutation. Biological significance and potential target for anti-cancer therapy. Annals of Oncology. 2001;12:745–760. doi: 10.1023/a:1011177318162. [DOI] [PubMed] [Google Scholar]
- 26.Bergstrom JD, Westermark B, Heldin NE. Epidermal growth factor receptor signaling activates met in human anaplastic thyroid carcinoma cells. Experimental Cell Research. 2000;259:293–299. doi: 10.1006/excr.2000.4967. [DOI] [PubMed] [Google Scholar]
- 27.Camp ER, Liu W, Fan F, Yang A, Somcio R, Ellis LM. RON, a tyrosine kinase receptor involved in tumor progression and metastasis. Annals of Surgical Oncology. 2005;12:273–81. doi: 10.1245/ASO.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Do SI, Jung WW, Kim HS, Park YK. The expression of epidermal growth factor receptor and its downstream signaling molecules in osteosarcoma. International Journal of Oncology. 2009;34:797–803. doi: 10.3892/ijo_00000205. [DOI] [PubMed] [Google Scholar]
- 29.Wen YH, Koeppen H, Garcia R, Chiriboga L, Tarlow BD, Peters BA, Eigenbrot C, Yee H, Steiner G, Greco MA. Epidermal growth factor receptor in osteosarcoma: expression and mutational analysis. Human Pathology. 2007;38:1184–1191. doi: 10.1016/j.humpath.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Freeman SS, Allen SW, Ganti R, Wu J, Ma J, Su X, Neale G, Dome JS, Daw NC, Khoury JD. Copy number gains in EGFR and copy number losses in PTEN are common events in osteosarcoma tumors. Cancer. 2008;113:1453–1461. doi: 10.1002/cncr.23782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kersting C, Gebert C, Agelopoulos K, Schmidt H, van Diest PJ, Juergens H, Winkelmann W, Kevric M, Gosheger G, Brandt B, Bielack S, Buerger H. Epidermal growth factor receptor expression in high-grade osteosarcomas is associated with a good clinical outcome. Clinical Cancer Research. 2007;13:2998–3005. doi: 10.1158/1078-0432.CCR-06-2432. [DOI] [PubMed] [Google Scholar]
- 32.Potratz JC, Saunders DN, Wai DH, Ng TL, McKinney SE, Carboni JM, Gottardis MM, Triche TJ, Jurgens H, Pollak MN, Aparicio SA, Sorensen PH. Synthetic lethality screens reveal RPS6 and MST1R as modifiers of insulin-like growth factor-1 receptor inhibitor activity in childhood sarcomas. Cancer Research. 2010;70:8770–8781. doi: 10.1158/0008-5472.CAN-10-1093. [DOI] [PubMed] [Google Scholar]
- 33.Khanna C, Prehn J, Hayden D, Cassaday RD, Caylor J, Jacob S, Bose SM, Hong SH, Hewitt SM, Helman LJ. A randomized controlled trial of octreotide pamoate long-acting release and carboplatin versus carboplatin alone in dogs with naturally occurring osteosarcoma: evaluation of insulin-like growth factor suppression and chemotherapy. Clinical Cancer Research. 2002;8:2406–2412. [PubMed] [Google Scholar]
- 34.Vail DM, Kurzman ID, Glawe PC, O'Brien MG, Chun R, Garrett LD, Obradovich JE, Fred RM, III, Khanna C, Colbern GT, Working PK. STEALTH liposome-encapsulated cisplatin (SPI-77) versus carboplatin as adjuvant therapy for spontaneously arising osteosarcoma (OSA) in the dog: a randomized multicenter clinical trial. Cancer Chemotherapy and Pharmacology. 2002;50:131–136. doi: 10.1007/s00280-002-0469-8. [DOI] [PubMed] [Google Scholar]
- 35.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Advances in Enzyme Regulation. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 36.Dussault I, Bellon SF. From concept to reality: the long road to c-Met and RON receptor tyrosine kinase inhibitors for the treatment of cancer. Anticancer Agents in Medicinal Chemistry. 2009;9:221–229. doi: 10.2174/187152009787313792. [DOI] [PubMed] [Google Scholar]
- 37.Albanell J, Gascon P. Small molecules with EGFR-TK inhibitor activity. Current Drug Targets. 2005;6:259–274. doi: 10.2174/1389450053765888. [DOI] [PubMed] [Google Scholar]
- 38.Frampton JE, Easthope SE. Gefitinib: a review of its use in the management of advanced non-small-cell lung cancer. Drugs. 2004;64:2475–2492. doi: 10.2165/00003495-200464210-00008. [DOI] [PubMed] [Google Scholar]
- 39.Freeman BB, III, Daw NC, Geyer JR, Furman WL, Stewart CF. Evaluation of gefitinib for treatment of refractory solid tumors and central nervous system malignancies in pediatric patients. Cancer Investigation. 2006;24:310–317. doi: 10.1080/07357900600632058. [DOI] [PubMed] [Google Scholar]
- 40.Chun R, de Lorimier LP. Update on the biology and management of canine osteosarcoma. The Veterinary Clinics of North America. Small Animal Practice. 2003;33:491–516. vi. doi: 10.1016/s0195-5616(03)00021-4. [DOI] [PubMed] [Google Scholar]
- 41.Dulak AM, Gubish CT, Stabile LP, Henry C, Siegfried JM. HGF-independent potentiation of EGFR action by c-Met. Oncogene. 2011;30:3625–3635. doi: 10.1038/onc.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Q, Seol DW, Carr B, Zarnegar R. Co-expression and regulation of Met and Ron proto-oncogenes in human hepatocellular carcinoma tissues and cell lines. Hepatology. 1997;26:59–66. doi: 10.1002/hep.510260108. [DOI] [PubMed] [Google Scholar]
- 43.Cheng HL, Liu HS, Lin YJ, Chen HH, Hsu PY, Chang TY, Ho CL, Tzai TS, Chow NH. Co-expression of RON and MET is a prognostic indicator for patients with transitional-cell carcinoma of the bladder. British Journal of Cancer. 2005;92:1906–1914. doi: 10.1038/sj.bjc.6602593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santoro MM, Collesi C, Grisendi S, Gaudino G, Comoglio PM. Constitutive activation of the RON gene promotes invasive growth but not transformation. Molecular and Cell Biology. 1996;16:7072–7083. doi: 10.1128/mcb.16.12.7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee CT, Chow NH, Su PF, Lin SC, Lin PC, Lee JC. The prognostic significance of RON and MET receptor coexpression in patients with colorectal cancer. Diseases of the Colon and Rectum. 2008;51:1268–1274. doi: 10.1007/s10350-008-9297-1. [DOI] [PubMed] [Google Scholar]
- 46.Lee WY, Chen HH, Chow NH, Su WC, Lin PW, Guo HR. Prognostic significance of co-expression of RON and MET receptors in node-negative breast cancer patients. Clinical Cancer Research. 2005;11:2222–2228. doi: 10.1158/1078-0432.CCR-04-1761. [DOI] [PubMed] [Google Scholar]
- 47.Puri N, Salgia R. Synergism of EGFR and c-Met pathways, cross-talk and inhibition, in non-small cell lung cancer. Journal of Carcinogenesis. 2008;7:9. doi: 10.4103/1477-3163.44372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mueller KL, Hunter LA, Ethier SP, Boerner JL. Met and c-Src cooperate to compensate for loss of epidermal growth factor receptor kinase activity in breast cancer cells. Cancer Research. 2008;68:3314–3322. doi: 10.1158/0008-5472.CAN-08-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mueller KL, Yang ZQ, Haddad R, Ethier SP, Boerner JL. EGFR/Met association regulates EGFR TKI resistance in breast cancer. Journal of Molecular Signaling. 2010;5:8. doi: 10.1186/1750-2187-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838–2849. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- 51.Seidman R, Gitelman I, Sagi O, Horwitz SB, Wolfson M. The role of ERK 1/2 and p38 MAP-kinase pathways in taxol-induced apoptosis in human ovarian carcinoma cells. Experimental Cell Research. 2001;268:84–92. doi: 10.1006/excr.2001.5262. [DOI] [PubMed] [Google Scholar]
- 52.Shukla A, Hillegass JM, MacPherson MB, Beuschel SL, Vacek PM, Pass HI, Carbone M, Testa JR, Mossman BT. Blocking of ERK1 and ERK2 sensitizes human mesothelioma cells to doxorubicin. Molecular Cancer. 9:314. doi: 10.1186/1476-4598-9-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale C-M, Zhao X, Christensen J, Kosaka T, Holmes AJ, Rogers AM, Cappuzzo F, Mok T, Lee C, Johnson BE, Cantley LC, Janne PA. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 54.Bean J, Brennan C, Shih JY, Riely G, Viale A, Wang L, Chitale D, Motoi N, Szoke J, Broderick S, Balak M, Chang WC, Yu CJ, Gazdar A, Pass H, Rusch V, Gerald W, Huang SF, Yang PC, Miller V, Ladanyi M, Yang CH, Pao W. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McDermott U, Pusapati RV, Christensen JG, Gray NS, Settleman J. Acquired resistance of non-small cell lung cancer cells to MET kinase inhibition is mediated by a switch to epidermal growth factor receptor dependency. Cancer Research. 2010;70:1625–1634. doi: 10.1158/0008-5472.CAN-09-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]