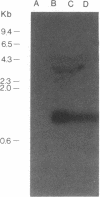
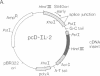
Abstract
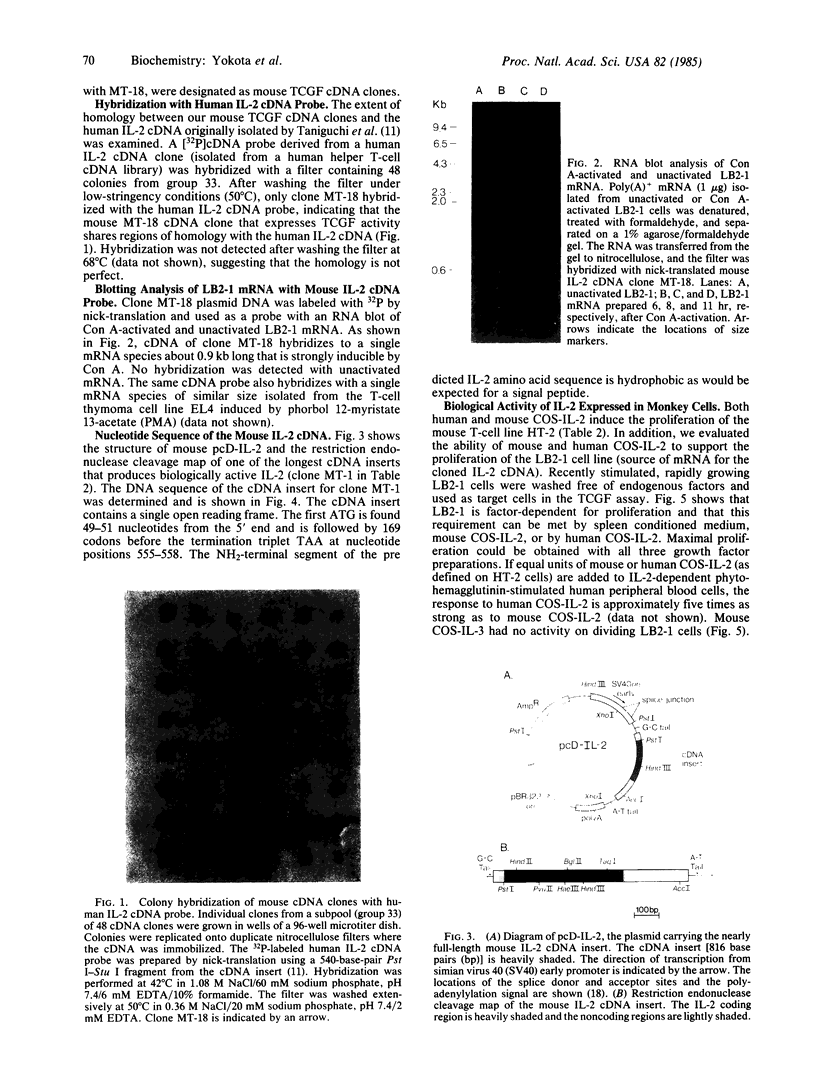
A cDNA sequence coding for mouse interleukin 2 (IL-2) has been cloned from a cDNA library prepared from mRNA derived from a concanavalin A-activated mouse T-cell clone. The library was constructed by using the pcD vector system, which permits the expression of cDNA inserts in mammalian cells. Screening of the library was performed by transfecting COS-7 monkey cells with pools of cDNA clones in order to express the products encoded by full-length cDNA inserts. By assaying the supernatant fluid, IL-2 cDNA clones that express T-cell growth-factor (TCGF) activity were identified. The DNA sequence codes for a polypeptide of 169 amino acid residues including a putative signal peptide. The mouse IL-2 amino acid sequence deduced from the nucleotide sequence of its cDNA shares extensive homology with the human IL-2 amino acid sequence reported previously. These results demonstrate that identification of full-length cDNA clones for many lymphokines may be achieved entirely on the basis of detection of the functional polypeptides in mammalian cells.
Full text
PDF
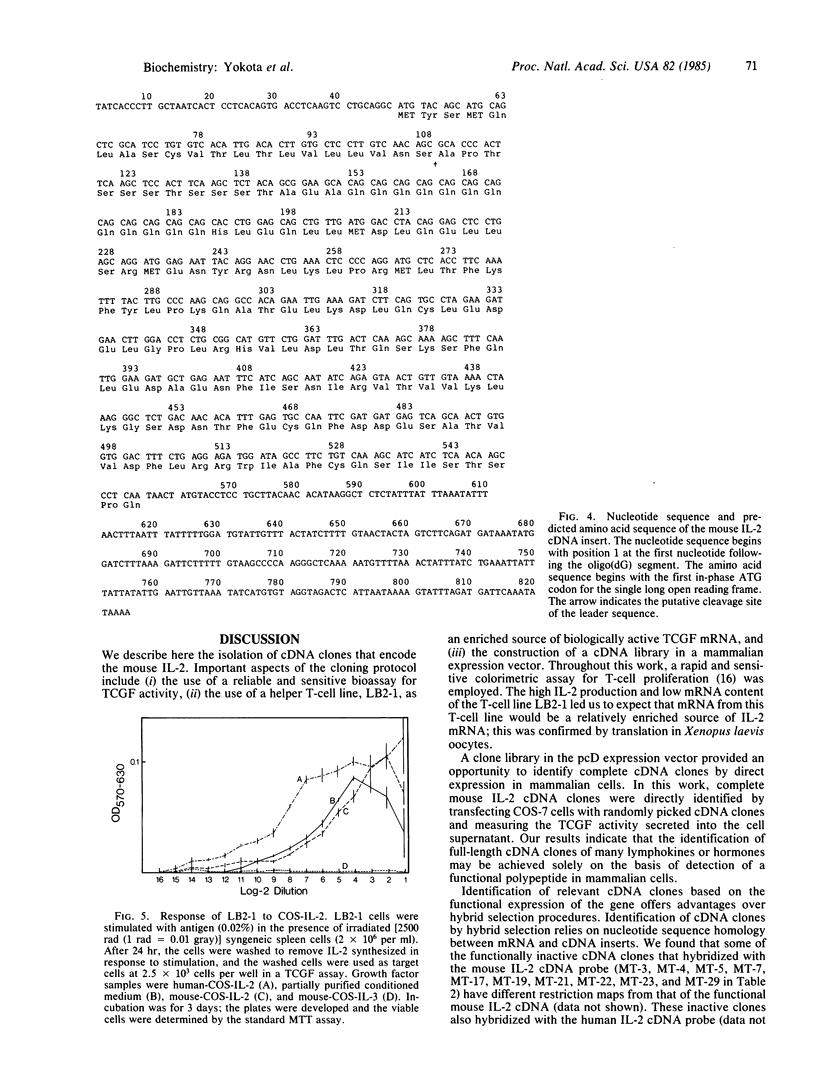

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brutlag D. L., Clayton J., Friedland P., Kedes L. H. SEQ: a nucleotide sequence analysis and recombination system. Nucleic Acids Res. 1982 Jan 11;10(1):279–294. doi: 10.1093/nar/10.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan B., Gibbs C., Paetkau V. Properties of sodium dodecyl sulfate-denatured Interleukin 2. J Immunol. 1981 Apr;126(4):1351–1354. [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sabato G., Chen D. M., Erickson J. W. Production by murine spleen cells of an activity stimulating the PHA-responsiveness of thymus lymphocytes. Cell Immunol. 1975 Jun;17(2):495–504. doi: 10.1016/s0008-8749(75)80053-0. [DOI] [PubMed] [Google Scholar]
- Fujita T., Takaoka C., Matsui H., Taniguchi T. Structure of the human interleukin 2 gene. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7437–7441. doi: 10.1073/pnas.80.24.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Gillis S., Smith K. A., Watson J. Biochemical characterization of lymphocyte regulatory molecules. II. Purification of a class of rat and human lymphokines. J Immunol. 1980 Apr;124(4):1954–1962. [PubMed] [Google Scholar]
- Gillis S., Watson J. Biochemical and biological characterization of lymphocyte regulatory molecules. V. Identification of an interleukin 2-producing human leukemia T cell line. J Exp Med. 1980 Dec 1;152(6):1709–1719. doi: 10.1084/jem.152.6.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mier J. W., Gallo R. C. Purification and some characteristics of human T-cell growth factor from phytohemagglutinin-stimulated lymphocyte-conditioned media. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6134–6138. doi: 10.1073/pnas.77.10.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nabel G., Galli S. J., Dvorak A. M., Dvorak H. F., Cantor H. Inducer T lymphocytes synthesize a factor that stimulates proliferation of cloned mast cells. Nature. 1981 May 28;291(5813):332–334. doi: 10.1038/291332a0. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paetkau V., Mills G., Gerhart S., Monticone V. Proliferation of murine thymic lymphocytes in vitro is mediated by the concanavalin A-induced release of a lymphokine (costimulator). J Immunol. 1976 Oct;117(4):1320–1324. [PubMed] [Google Scholar]
- Rubin C. M., Schmid C. W. Pyrimidine-specific chemical reactions useful for DNA sequencing. Nucleic Acids Res. 1980 Oct 24;8(20):4613–4619. doi: 10.1093/nar/8.20.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnetzler M., Oommen A., Nowak J. S., Franklin R. M. Characterization of chicken T cell growth factor. Eur J Immunol. 1983 Jul;13(7):560–566. doi: 10.1002/eji.1830130709. [DOI] [PubMed] [Google Scholar]
- Shaw J., Monticone V., Paetkau V. Partial purification and molecular characterization of a lymphokine (costimulator) required for the mitogenic response of mouse thymocytes in vitro. J Immunol. 1978 Jun;120(6):1967–1973. [PubMed] [Google Scholar]
- Stern A. S., Pan Y. C., Urdal D. L., Mochizuki D. Y., DeChiara S., Blacher R., Wideman J., Gillis S. Purification to homogeneity and partial characterization of interleukin 2 from a human T-cell leukemia. Proc Natl Acad Sci U S A. 1984 Feb;81(3):871–875. doi: 10.1073/pnas.81.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T., Matsui H., Fujita T., Takaoka C., Kashima N., Yoshimoto R., Hamuro J. Structure and expression of a cloned cDNA for human interleukin-2. Nature. 1983 Mar 24;302(5906):305–310. doi: 10.1038/302305a0. [DOI] [PubMed] [Google Scholar]
- Watson J. Continuous proliferation of murine antigen-specific helper T lymphocytes in culture. J Exp Med. 1979 Dec 1;150(6):1510–1519. doi: 10.1084/jem.150.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Gillis S., Marbrook J., Mochizuki D., Smith K. A. Biochemical and biological characterization of lymphocyte regulatory molecules. I. Purification of a class of murine lymphokines. J Exp Med. 1979 Oct 1;150(4):849–861. doi: 10.1084/jem.150.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Lee F., Rennick D., Hall C., Arai N., Mosmann T., Nabel G., Cantor H., Arai K. Isolation and characterization of a mouse cDNA clone that expresses mast-cell growth-factor activity in monkey cells. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1070–1074. doi: 10.1073/pnas.81.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]