Figure 3.
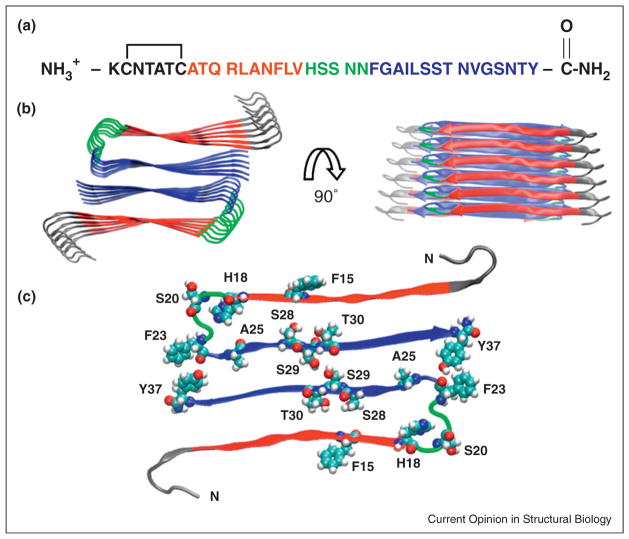
The structure of human IAPP: (a) The primary sequence of human IAPP. Residues 8–17 form intermolecular β-sheets in models of the IAPP amyloid fiber and are color coded in red. Residues 18–22, colored in green, form a bend in the UCLA model while residues 23–37, colored in blue, take part in an intermolecular β-sheet. The color coding corresponds to the IAPP amyloid structural model developed by the UCLA group [23]. The first 7 residues do not take part in β-sheet structure in existing models of IAPP amyloid. (b) A structural model of the IAPP fiber developed by the UCLA group [23]. Two views are shown: a top down view and an image rotated by 90 degrees. The color coding corresponds to that used in panel A. (c) A view of one layer of the stacked structure shown in panel (b). His-18, Ser-20, Ser-28, Ser-29, Thr-30 are shown in space-filling representation. The protonation state of His-18 affects the rate of amyloid formation [77]. A Ser-20 to Gly mutant is the only reported mutation in mature IAPP found in humans and has been shown to accelerate amyloid formation [4,11]. Ser-28, Ser-29 and Thr-30 form key inter-peptide contacts in the UCLA model. IAPP contains three aromatic residues (Phe-15, Phe-23, and Tyr-37) which are also highlighted.