Figure 1.
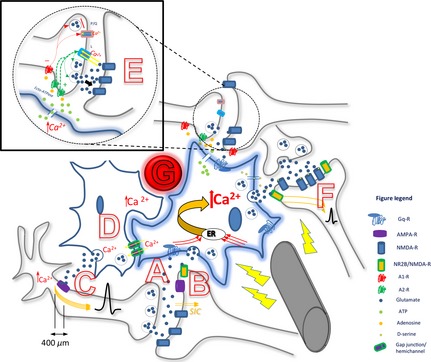
Summary diagram depicting the proposed simultaneity of deep brain stimulation (DBS) effects on axons, astrocyte–astrocyte and astrocyte–neuronal interactions. DBS electrode is here activating one local astrocyte (blue), leading to increased intracelluar Ca2+, causing hypermorphism and release of gliotransmitters, resulting in increased axonal flow and synaptic transmission. This astrocyte has multiple processes, each intimately participating at specific neuronal synapses; activation at one process/micro‐domain may or may not spread to others. Multiple processes are likely occurring simultaneously. (A) Secondary activation by DBS: Increased synaptic activity between axon and dendrite, with release of neurotransmitters (e.g., glutamate), is sensed by the astrocytic Gq protein‐coupled receptors on a process at the tripartite synapse (such as mGluR5), resulting in an increase in intracelluar Ca2+. This activation can be confined to one particular micro‐domain, or can be distributed throughout the astrocyte to all its processes. Each micro‐domain can express different gliotransmitters particular to the synapse. (B) Increased Ca2+ in astrocytes evokes postsynaptic slow inward currents (SICs) in nearby neurons through astrocytic glutamate release, which binds to the NR2B subunit of extrasynaptic NMDA‐R and increases neuronal excitability and increases probability of neurotransmitter release from neuronal presynaptic terminals. (C) Ca2+ activation in astrocyte causes release of extra‐synaptic glutamate; glutamate binds to peri‐synaptic AMPA‐R on presynaptic neuronal processes within 400 μm of axon hillock, causes influx of Ca2+ which broadens action potential (AP). (D) Astrocytes in clusters, coactivated and synchronized via gap junctions/hemichannels. (E) Astrocytic processes detect local synaptic activity – glutamate release – which causes a local Ca2+ evoked response mediated through mGluR5 activation; this leads to release of glial ATP which is is converted by ecto‐ATPase into adenosine. Adenosine then acts at presynaptic A1 coupled P/Q‐type calcium channels to incur synaptic depression while it also acts at presynaptic A2 coupled L‐type calcium channels to incur synaptic potentiation. Relative amounts of synaptic adenosine as well as interactions between each receptor determine the balance; here potentiation is depicted. Also, activation of presynaptic adenosine A2A receptors increases basal synaptic transmission. (F) Different gliotransmitters can be released at other processes due to Ca2+ activation in astrocyte. D‐serine and glutamate are released by the activated astrocyte, modulate NMDA‐R activity and synaptic plasticity. Glutamate released due to DBS astrocytic activation acts on neuronal extrasynaptic NR2B/NMDA‐R, causing increased neuronal excitability and probability of downstream synaptic transmission. (G) Blood vessel proximity to astrocytes – increased CBF linked to state changes of astrocyte activity; DBS induced changes in CBF and interaction with astrocytes not resolved.
