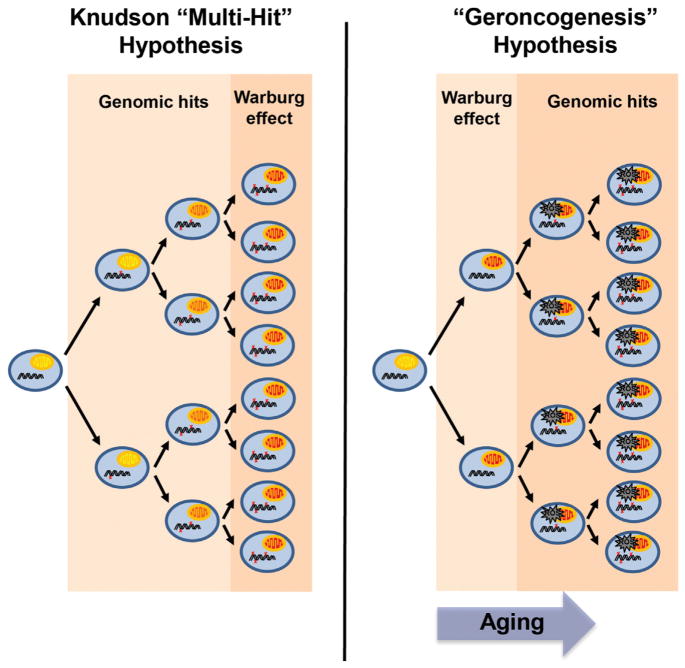
Figure 1. The Geroncogenesis Hypothesis: Aging-Induced Metabolic Decline as a Driver of Tumorigenesis.
According to the Knudson, or “multi-hit,” hypothesis, cancer occurs more frequently as we age because time is necessary for cells to acquire genetic mutations that drive carcinogenesis (indicated by red “x”). According to this model, the shift to oxidative glycolysis, known as the Warburg effect, occurs subsequent to these early hits. An alternative possibility is that the natural decline in oxidative metabolism as we age induces a Warburg-like metabolic state in normal tissues (indicated by red mitochondria). This increases the ROS production and sets the metabolic stage for later mutations to drive tumorigenesis. Low-calorie diets, exercise, and CR mimetic compounds delay this metabolic shift, thereby reducing the chance that oncogenic mutations will occur in a cell with optimal metabolism for tumorigenesis. The model predicts that CR mimetics could be used to reverse the metabolic reprogramming of tumors and even to prevent aging tissues from undergoing this switch in the first place.