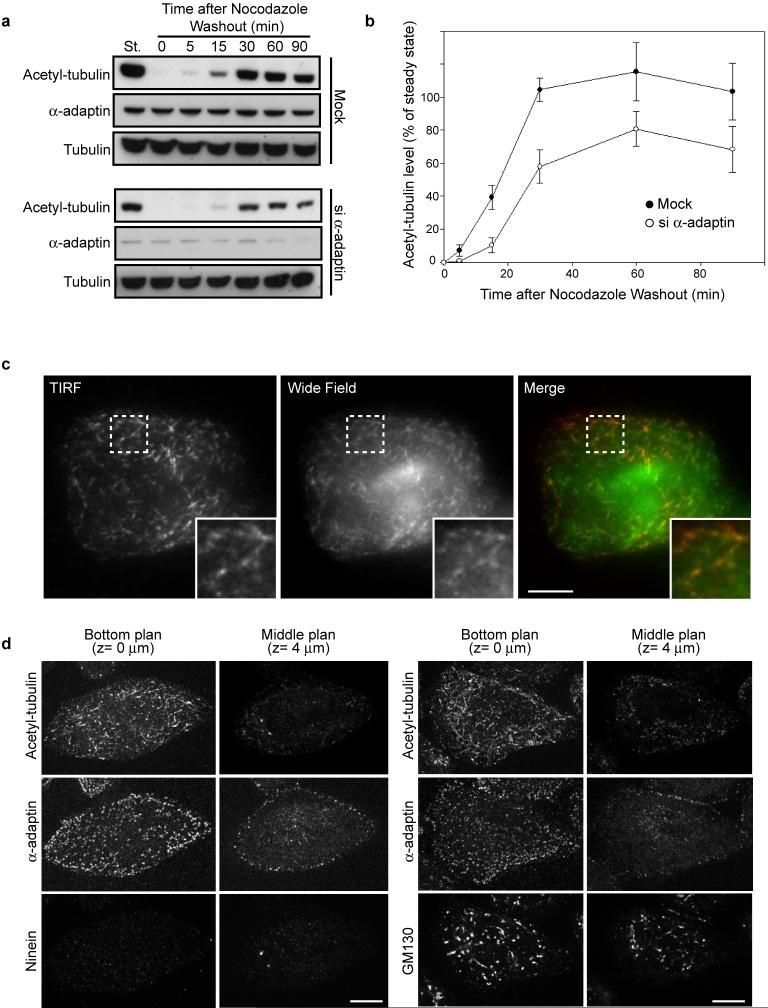
Extended Data Figure 8. Kinetics of acetylated-microtubule recovery at the plasma membrane after nocodazole washout.
a, HeLa cells transfected with siNT (mock) or α-adaptin siRNA were treated with nocodazole for 5 hours and then the drug was washed out to allow the microtubules to repolymerize for the indicated times. Cell lysates were analyzed by western-blot using the indicated antibodies. St, steady state situation without nocodazole treatment. b, Quantification of the pixel densities of the bands detected by the anti-K40 acetyl-tubulin antibody as in (a) expressed as a percentage of respective steady state levels ± s.e.m (normalized to total tubulin levels, from three independent experiments). c, HeLa cells were treated with nocodazole for 5 hours and then the drug was washed out to allow microtubules to re-polymerize for 5 min before fixation and staining with anti-K40 acetyl-tubulin antibody. Cells were then imaged in TIRF or wide-field mode to visualize acetylated microtubules in the vicinity of the adherent plasma membrane or within the cytoplasm, respectively. Note that the pattern of acetylated microtubules does not differ between TIRF and wide-field image indicating that most of acetylated microtubules are in close proximity to the plasma membrane. Insets show the boxed region at higher magnification. Scale bar, 10 μm. d, HeLa cells were treated with nocodazole for 5 hours and then the drug was washed out to allow microtubules to re-polymerize for 5 min before fixation and staining with indicated antibodies. Images were acquired with a spinning disk microscope by focusing on the adherent plasma membrane (bottom plane, z-section= 0 μm) or in the middle of the cell (middle plane, z-section= 4 μm). Note that most of the acetylated microtubule segments are found in the vicinity of the plasma membrane while not all the intracellular organelles (Ninein-positive centrosomes, GM130-associated Golgi stacks) are at the bottom of the cell.