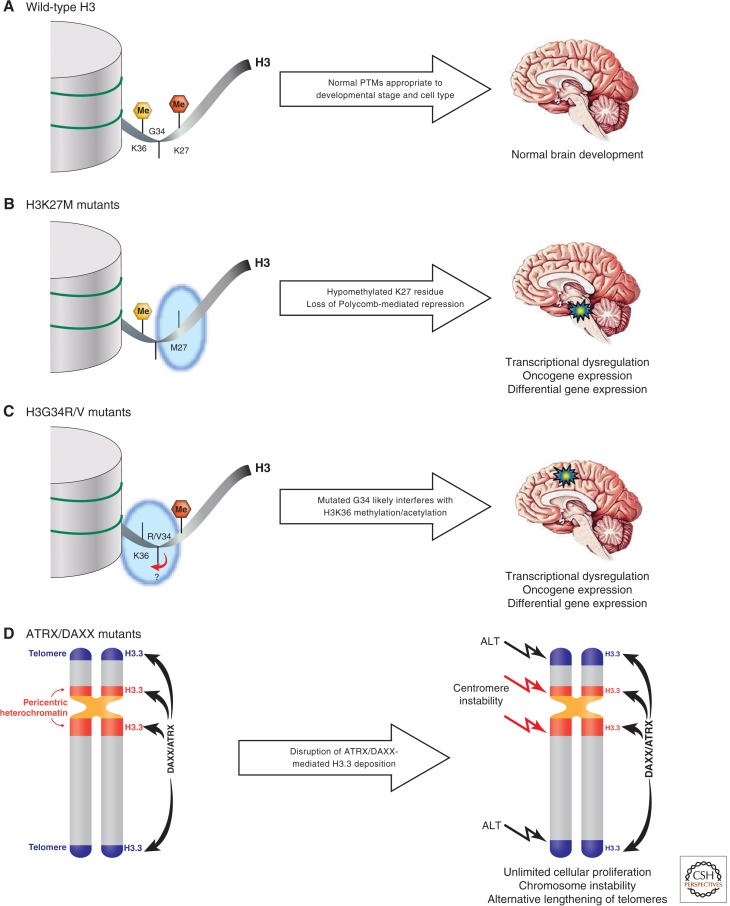
Figure 1.
Potential effects of histone H3 and ATRX/DAXX mutations in the tumorigenic process of pediatric high-grade gliomas. (A) In the absence of mutations, the amino-terminal tail of histone H3 receives a myriad of cell-type- and developmental-stage-specific posttranslational modifications (PTMs) dictating the gene expression profiles required for normal brain development. (B) The H3K27M mutation results in a hypomethylated K27 residue (loss of red hexagon), preventing Polycomb-mediated repression of target genes. This likely results in oncogene expression and an altered gene expression profile suitable for the development of high-grade gliomas of the midline structures, most notably diffuse intrinsic pontine gliomas (blue/yellow star). (C) The H3G34R/V mutation diminishes the levels of H3K36 methylation (loss of yellow hexagon), which may impact transcriptional elongation in addition to generating a gene expression profile conducive to the formation of non-brainstem pediatric glioblastomas (blue/yellow star). The H3G34R/V may also impact on the acetylation of H3K36 (not shown for illustrative purposes). (D) Mutations in ATRX/DAXX alter the proper deposition of histone H3.3 at pericentric and telomeric heterochromatic loci, thus compromising chromatin structure and allowing for genomic instability and alternative lengthening of telomeres (ALT). H3K27me, red hexagon; H3K36me, yellow hexagon; high-grade glioma, blue/yellow star; telomeres, blue chromatin shading; pericentric heterochromatin, red chromatin shading.