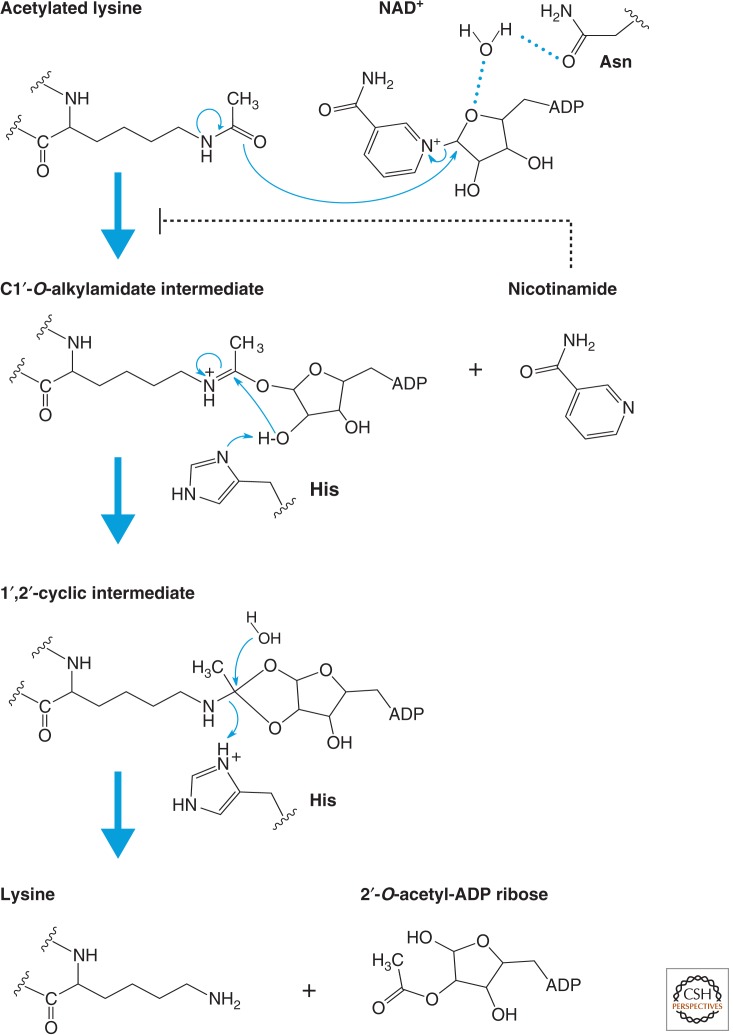
Figure 5.
Catalytic mechanism of sirtuins (Class III). Proposed mechanism of the NAD+-dependent deacetylase reaction. The first step of the reaction involves nucleophilic addition of the acetamide oxygen to the C1′ position of the nicotinamide ribose to form a C1′-O-alkylamidate intermediate and free nicotinamide. Next, the 2′-hydroxy group of the NAD+ ribose is activated by an active site histidine residue that, in turn, attacks the C1′-O-alkylamidate to form the 1′,2′-cyclic intermediate. The 1′,2′-cyclic intermediate is then attacked by an activated water molecule resulting in the formation of deacetylated lysine and 2′-O-acetyl-ADP ribose. 2′-O-acetyl-ADP ribose can be readily converted to 3′-O-acetyl-ADP ribose in aqueous solution by nonenzymatic intramolecular transesterification. Thus, nicotinamide, the deacetylated peptide, and a mixture of 2′- and 3′-O-acetyl-ADP ribose are the final reaction products.