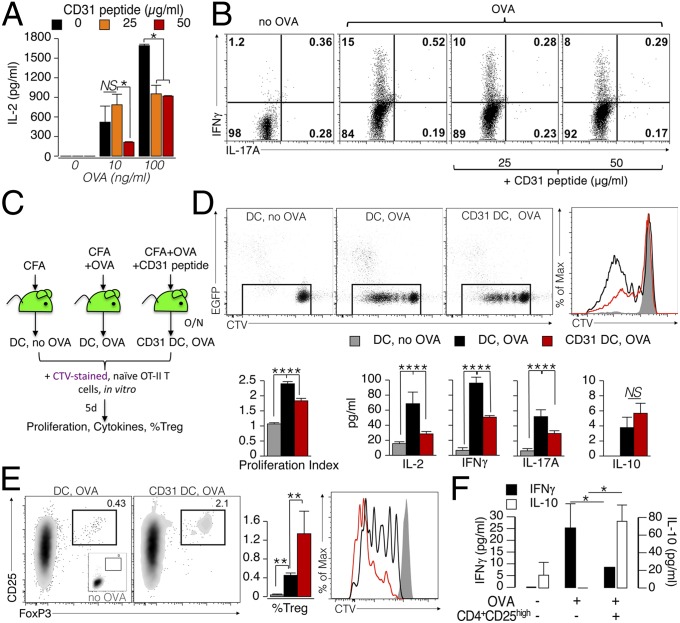
Fig. 6.
CD31 signaling favors the development of tolerogenic DCs. (A) BMDCs (1 × 105) were matured overnight with LPS (100 ng/mL) in the presence of the CD31 peptide (25 or 50 μg/mL). OVA peptide (10 or 100 ng/mL) was added during the last 4 h of LPS stimulation. The DCs were then washed and cocultured with naive (CD62L+) OT-II CD4+ T cells (5 × 105) for 4 d. A dose-dependent inhibition of IL-2 production by OT-II CD4+ T was exerted by CD31 peptide-conditioned DCs on cognate T cells. *P < 0.05. (B) Representative dot plots display cytometric data of intracellular IFN-γ and IL-17A cytokine staining. Intracellular IFN-γ, which was readily increased in OT-II CD4+ T cells stimulated with OVA-loaded DCs, was reduced in a dose-dependent manner when the antigen was presented by CD31-conditioned DCs (DCs that had loaded the OVA antigen in the presence of the CD31 peptide). (C–F) Evaluation of the effect of CD31 signaling on DCs in vivo. (C) Experimental strategy (n = 3–5 per group). (D) Evaluation of CTV dilution showed that CD31 conditioning reduces the ability of DCs to elicit the proliferation of T cells. These results were confirmed by the lower production of IL-2, IFN-γ, and IL-17A, but not of IL-10. **P < 0.01. (E) CD31-conditioned DCs favored the differentiation and proliferation (CTV dilution) of CD4+ Tregs (CD25+FoxP3+) from naive OT-II CD4+ T cells. (F) Newly generated Tregs were fully competent in suppressing the production of effector cytokines by OT-II cells at a ratio of 1:10. Of note, the presence of these Tregs induced an increase in the amount of soluble IL-10. *P < 0.05.