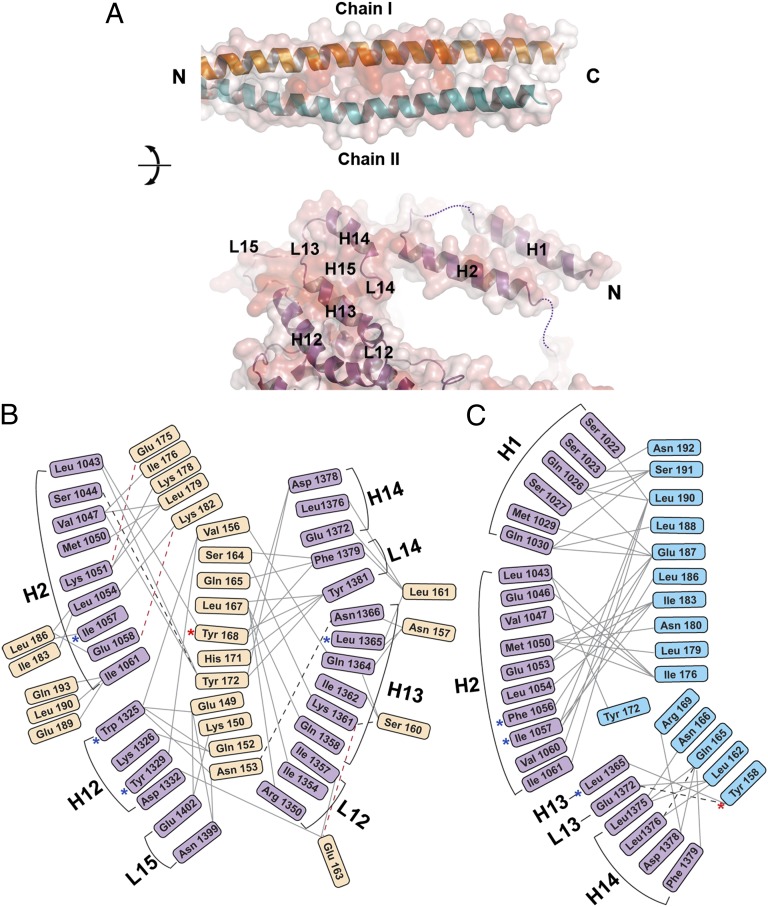
Fig. 3.
Residue-by-residue contacts in the *Myo4p-She3p heterotrimer. (A) Interfaces between She3p coiled-coil region and *Myo4p are shown in an open-book depiction, and their transparent surface representations are colored according to sequence conservation, from white (0%) to red (100%) identity. (Upper) The two chains of She3p are labeled as chain I (orange) and II (blue). (Lower) Helices and loops of *Myo4p are marked and numbered; disordered parts of loops are indicated by dashed lines (Figs. S2A, S3A, and S4B). (B and C) Interactome of residues of *Myo4p and the two She3p chains: chain I (Glu-149–Gln-193) (Left), and She3p chain II (Tyr-158–Asn-192) (Right). Solid gray lines represent hydrophobic Van der Waal interactions, dashed black lines indicate hydrogen bonds, and red dashed lines depict electrostatic interactions. Note that two She3p chains interact asymmetrically with *Myo4p (Figs. S2A, S3A, and S4). Mutations reported previously are indicated by blue asterisks, and mutations studied in this work are indicated by red asterisks (25).