Fig. 4.
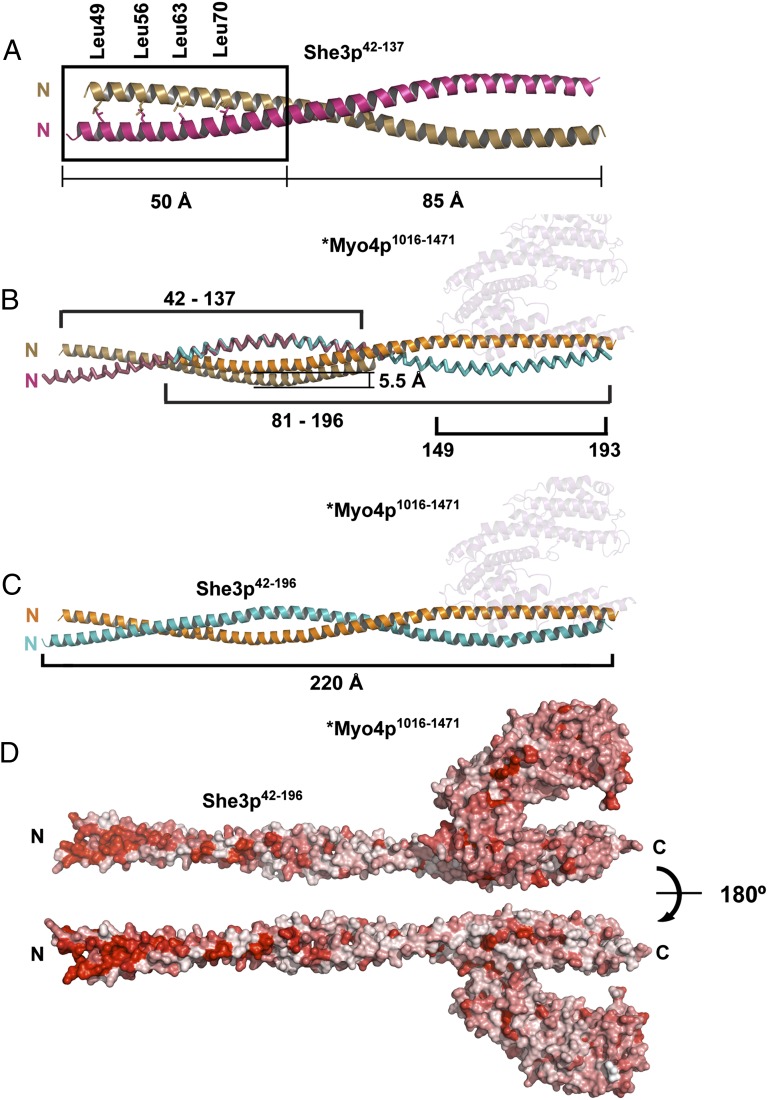
Crystal structure of an upstream section of the She3p coiled coil reveals a highly conserved surface region. (A) She3p42-137 was separately crystallized (Fig. 1 and Fig. S3 A and B) and formed a 135-Å-long, parallel coiled coil with a classical Leucine Zipper at its N-terminal end (the four Leucines of the zipper are indicated as sticks). The two She3p42-137 helices are colored brown and pink. The upstream Leucine Zipper region of She3p is boxed. (B) Overlap of the coiled-coil region of She3p42-137 (A) and the She3p moiety of *Myo4p1016-1471•She3p81-196 (Fig. 2A). In their superimposed form, one of the She3p42-137 ribbons is displaced by 5.5 Å. The position of *Myo4p1016-1471 is indicated in pale cyan. (C) A 220-Å-long composite structure of She3p42-196 in complex with *Myo4p1016-1471 (pale cyan) is indicated in the color code of Fig. 2A. (D) Surface representation of a composite *Myo4p1016-1471•She3p42-196 complex. Sequence conservation is colored from white (0%) to red (100%) identity. Note high degree of surface conservation at the N-terminal Leucine zipper region of She3p. We propose this region of She3p to be a candidate for binding to a not yet identified ligand of tubular endoplasmic reticulum (tER) (Fig. 6B).