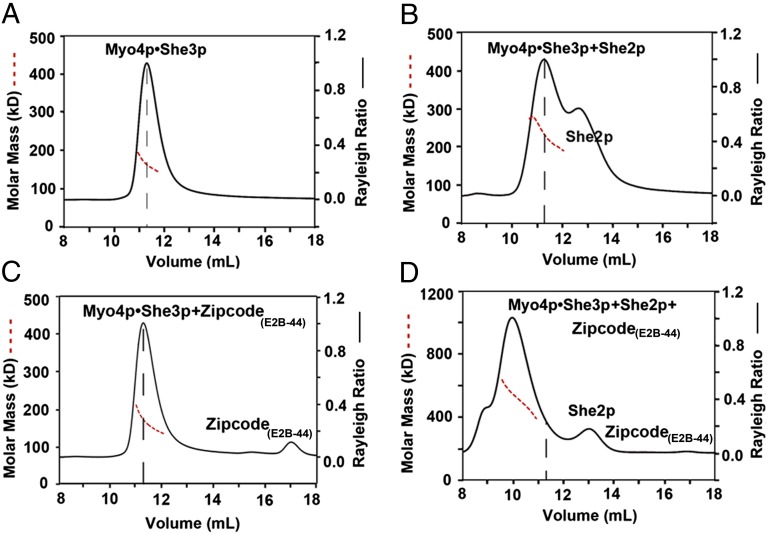
Fig. 5.
Coupling of two Myo4p•She3p heterotrimers by zipcode oligonucleotide and She2p. Indicated components were incubated and subsequently analyzed by size-exclusion chromatography coupled to MALS, plotted as Fig. 1B. (A) Peak of wtMyo4p918-1471•She3pFL (340 µg of protein at 1.7 mg/mL) elutes with a molar mass of 165.6 ± 0.3 kDa (dashed red line), close to the expected molar mass of 162.9 kDa for a 1:2 heterotrimer; the peak position in the elution profile is marked with a vertical dashed line (black) in this panel and the following panels. (B) As A, but after incubation with full-length She2pFL (680 µg of protein at 1.7 mg/mL in a molar ratio of Myo4p:She3p:She2p of 1:2:4); peak elutes with a molar mass of 237.4 ± 0.5 kDa, indicating a dynamic equilibrium between Myo4p•She3p and a She2p tetramer. (C) As A, but after incubation with oligonucleotide E2B-44 (340 µg of protein at 1.7 mg/mL and 29.2 µg of E2B-44 at 0.146 mg/mL; molar ratio of Myo4p:She3p:E2B-44 1:2:1). The peak elutes at a molar mass of 163.8 ± 0.65 kDa (dashed red line) but shows a slightly higher molar mass at the leading edge of the peak, consistent with some zipcode oligonucleotide binding to Myo4p•She3p heterotrimer. (D) As in B, but after incubation with 44-nt-long, zipcode RNA (E2B-44) (total 680 µg of protein mixture at 1.7 mg/mL with 101 µg of E2B-44 at 0.252 mg/mL; molar ratio of Myo4p:She3p:She2p:E2B-44 is 1:2:4:3); peak elutes at a molar mass of 467.2 ± 1.2 kDa, consistent with coupling of two Myo4p•She3p heterotrimers by one She2p tetramer and a single E2B-44 oligonucleotide in a molar ratio of 2:4:4:1 (calculated molar mass: 457.1 kDa). However, because of the small mass contribution of the oligonucleotide, we cannot rule out that more than one oligo bound.