Fig. 2.
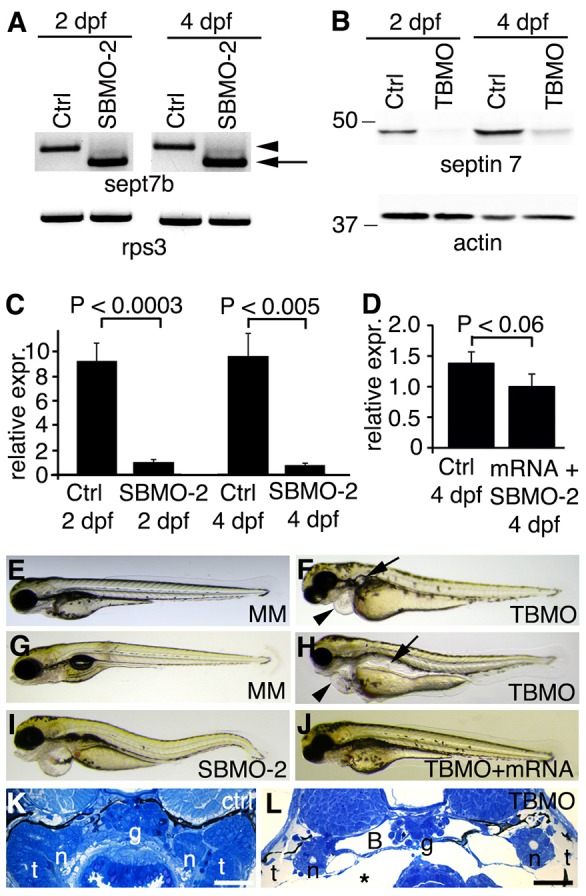
sept7b knockdown causes body curvature, edema and pronephric cysts. (A) PCR confirms skipping of exon 2 (arrow; amplicon 626 bp) of sept7b in SBMO-2-injected embryos at 2 and 4 dpf. Arrowhead marks the non-spliced sept7b amplicon (726 bp). Rps3 is used as a control (amplicon 428 bp). Ctrl, control. (B) Immunoblotting of 2- and 4-dpf larvae that had been injected with TBMO indicates that TBMO efficiently blocked translation of sept7b mRNA. Actin is used as a loading control. (C) qRT-PCR indicates that the expression level of sept7b is reduced by 89–92% in SBMO-2-injected embryos at 2 and 4 dpf. Rps3 was used to normalize expression values. (D) qRT-PCR of 4-dpf larvae shows that the expression level of sept7b is partially restored in rescue experiments when capped zebrafish sept7b mRNA is co-injected with the sept7b SBMO-2. (E,F) Injection of the sept7b TBMO into embryos causes pericardial (arrowhead) and yolk sac edema, and pronephric cysts (arrow) at 3 dpf (F), whereas the TBMO-MM-injected embryos (MM) do not show phenotypic differences (E). (G,H) sept7b TBMO-injected larvae (H) show pericardial (arrowhead) and yolk sac edema, body axis curvature, and dilation of pronephric tubules (arrow) compared to the TBMO-MM-injected larvae at 4 dpf. (I) sept7b SBMO-2 injection causes pericardial edema and body axis curvature. (J) The phenotypic differences can be rescued by co-injection of sept7b capped mRNA with the sept7b TBMO. (K) Histological section of a 4-dpf control larvae. (L) sept7b morphant larvae at 4 dpf shows a dilated Bowman's space (B). g, glomerulus; n, neck segment of the pronephric tubule; t, pronephric tubule; asterisk, edema. Scale bars: 50 µm.
